Abstract
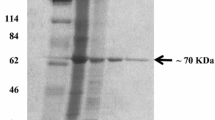
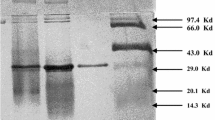
Thermostable N-acylamino acid recemase from Amycolatopsis sp. TS-1-60, a rare actinomycete strain selected for its ability to grow on agar plates incubated at 40° C, was purified to homogeneity and characterized. The relative molecular mass (M r) of the native enzyme and the subunit was estimated to be 300 000 and 40 000 on gel filtration chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis respectively. The isoelectric point (pI) of the enzyme was 4.2. The optimum temperature and pH were 50° C and 7.5 respectively. The enzyme was stable at 55° C for 30 min. The enzyme catalyzed the racemization of optically active N-acylamino acids such as N-acetyl-l-or d-methionine, N-acetyl-l-valine, N-acetyl-l-tyrosine and N-chloroacetyl-l-valine. In addition, the enzyme also catalyzed the recemization of the dipeptide l-alanyl-l-methionine. By contrast, the optically active amino acids, N-alkyl-amino acids and methyl and athyl ester derivatives of N-acetyl-d- and l-methionine were not racemized. The apparent K m values for N-acetyl-l-methionine and N-acetyl-d-methionine were calculated to be 18.5 mM and 11.3 mM respectively. The enzyme activity was markedly enhanced by the addition of divalent metal ions such as Co2+, Mn2+ and Fe2+ and was inhibited by addition of EDTA and P-chloromercuribenzoic acid. The similarity between the NH2-terminal amino acid sequence of the enzyme and that of Streptomyces atratus Y-53 [Tokuyama et al. (1994) Appl Microbiol Biotechnol 40:835–840] was above 80%.
Similar content being viewed by others
References
Adams E (1972) Amino acid racemases and epimerases. In: Boyer PD (ed) The enzyme, vol. 6. Academic Press, New York London, pp 470–508
Behrman E J (1962) Tryptophan metabolism in Pseudomonas. Nature 196:150–152
Cardinale GJ, Abeles RH (1968) Purification and mechanism of action of proline racemase. Biochemistry 7:3970–3978
Esaki N, Walsh CT (1986) Biosynthetic alanine racemase of Salmonella typhimurium: purification and characterization of the enzyme encoded by the alr gene. Biochemistry 25:3261–3267
Fee JA, Hegeman GD, Kenyon GI (1974) Mandelate racemase from Pseudomonas putida. Subunit composition and absolute divalent metal ion requirement. Biochemistry 13:2528–2532
Inagaki K, Tanizawa K, Tanaka H, Soda K (1987) Purification and characterization of amino acid racemase with very broad substrate specificity from Aeromonas caviae. Agric Biol Chem 51:173–180
Nakajima N, Tanizawa K, Tanaka H, Soda K (1988) Distribution of glutamate racemase in lactic acid bacteria and further characterization of the enzyme from Pediococcus pentosaceus. Agric Biol Chem 52:3099–3104
Okuda H, Yohda M, Giga-Hama Y, Ueno Y, Ohdo S, Kumagai H (1991) Distribution and purification of aspartate racemase in lactic acid bacteria. Biochim Biophys Acta 1078:377–382
Roise D, Soda K, Yagi T, Walsh CT (1984) Inactivation of the Pseudomonas striata broad specificity amino acid racemase by D and L isomers of β-subunited alanines: kinetics, stoichiometry, active site peptide, and mechanistic studies. Biochemistry 23:5195–5201
Rosso G, Takahashi K, Adams E (1969) Coenzyme component of purified alanine racemase from Pseudomonas. Biochem Biophys Res Commun 34:134–140
Soda K, Osumi T (1969) Crystalline amino acid rasemase with low substrate specificity Biochem Biophys Res Commun 35:363–368
Spackman DH, Stein WH, Moore S (1958) Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem 30:1190–1206
Tokuyama S, Hatano K, Takahashi T (1994a) Discovery of a novel enzyme, N-acylamino acid racemase in an actinomycete: screening, isolation, and identification. Biosci Biotechnol Biochem 58:24–27
Tokuyama S, Miya H, Hatano K, Takahashi T (1994b) Purification and properties of a novel enzyme, N-acylamino acid racemase, from Streptomyces atratus Y-53. Appl Microbiol Biotechnol 40:835–840
Tosa T, Mori T, Fuse N, Chibata I (1967) Studies on continuous enzyme reaction. IV. Preparation of a DEAE-Sephadex-aminoacylase column and continuous optical resolution of acyl-dl-amino acids. Biotechnol Bioeng 9:603–615
Wiseman JS, Nichols JS (1984) Purification and properties of diaminopimelic acid epimerase from Escherichia coli. J Biol Chem. 259:890–891
Yamauchi T, Choi S-Y, Okada H, Yohda M, Kumagai H, Esaki N, Soda K (1991) Properties of aspartate racemase, a pyridoxal 5′-phosphate independent amino acid racemase. J Biol Chem 267:18361–18364
Yorifuji T, Ogata K, Soda K (1971) Arginine racemase of Pseudomonas graveolens. I. Purification crystallization, and properties. J Biol Chem 246:5085–5092
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tokuyama, S., Hatano, K. Purification and properties of thermostable N-acylamino acid racemase from Amycolatopsis sp. TS-1-60. Appl Microbiol Biotechnol 42, 853–859 (1995). https://doi.org/10.1007/BF00191181
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191181