Abstract
The frequency distribution of the 8-h urinary ratio of log metoprolol/α-hydroxymetoprolol was assessed in 65 healthy, unrelated Jordanian volunteers.
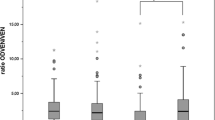
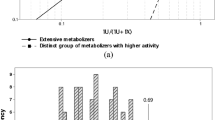
There was no apparent bimodality in the frequency distribution of this ratio among the subjects studied. The frequency of the poor metabolizer phenotype of metoprolol α-hydroxylation was 1.5% (one subject). There was a significant correlation (r=0.61, P<0.05, n=39) between the log metoprolol/α-hydroxymetoprolol and the log debrisoquine/4-hydroxydebrisoquine ratios. However, the frequency of poor metabolizer status of debrisoquine among the 39 subjects was 7.7% (three subjects). Only one of the poor metabolizers of debrisoquine was also a poor metabolizer of metoprolol α-hydroxylation.
These findings indicate that metoprolol α-hydroxylation by CYP2D6 represents a poor probe for studying debrisoquine polymorphism in Jordanians.
Similar content being viewed by others
References
Cholerton S, Daly AK, Idle JR (1992) The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci 13: 434–439
Eichelbaum M, Gross AS (1990) The genetic polymorphism of debrisoquine/sparteine metabolism — clinical aspects. Pharmacol Ther 46: 377–394
Hadidi HF, Cholerton S, Monkman SC, Armstrong M, Irshaid YM, Rawashdeh NM, Daly AK, Idle JR (1994) Debrisoquine 4-hydroxylation (CYP2D6) polymorphism in Jordanians. Pharmacogenetics 4: 159–161
Horai Y, Ishizaki T, Kusaka M, Tsujimoto G, Hashimoto K (1988) Simultaneous determination of metoprolol and α-hydroxymetoprolol in human plasma and urine by liquid chromatography with a preliminary observation on metoprolol oxidation in Japanese subjects. Ther Drug Monit 10: 428–433
Horai Y, Ishizaki T, Ishikawa K (1988) Metoprolol oxidation in a Japanese population:evidence for only one poor metabolizer among 262 subjects. Br J Clin Pharmacol 26: 807–808
Iyun AO, Lennard MS, Tucker GT, Woods HF (1986) Metoprolol and debrisoquine metabolism in Nigerians: lack of evidence for polymorphic oxidation. Clin Pharmacol Ther 40: 387–394
Lennard MS (1985) Quantitative analysis of metoprolol and three of its metabolites in urine and liver microsomes by high-performance liquid chromatography. J Chromatogr 342: 199–205
Lennard MS, Silas JH (1983) Rapid determination of metoprolol and α-hydroxymetoprolol in human plasma and urine by high-performance liquid chromatography. J Chromatogr 272: 205–209
Lennard MS, Silas JH, Freestone S, Trevethick J (1982) Defective metabolism of metoprolol in poor hydroxylators of debrisoquine. Br J Clin Pharmacol 14: 301–303
Lennard MS, Tucker GT, Silas JH, Woods HF (1986) Debrisoquine polymorphism and the metabolism and action of metoprolol, timolol, propranolol and atenolol. Xenobiotica 16: 435–447
Lennard MS, Iyun AO, Jackson PR, Tucker GT, Woods HF (1992) Evidence for a dissociation in the control of sparteine, debrisoquine and metoprolol metabolism in Nigerians. Pharmacogenetics 2: 89–92
McGourty JC, Silas JH, Lennard MS, Tucker GT, Woods HF (1985) Metoprolol metabolism and debrisoquine oxidation polymorphism — population and family studies. Br J Clin Pharmacol 20: 555–566
Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF, Loper JC, Sato R, Waterman MR, Waxman DJ (1991) The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol 10: 1–14
Price-Evans DAP, Mahgoub A, Sloan TP, Idle JR, Smith RL (1980) A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet 17: 102–105
Simooya OO, Njunju E, Hodjegan AR, Lennard MS, Tucker GT (1993) Debrisoquine and metoprolol oxidation in Zambians: a population study. Pharmacogenetics 3: 205–208
Smith DA, Jones BC (1992) Speculations on the substrate structure-activity relationship (SSAR) of cytochrome P450 enzymes. Biochem Pharmacol 44: 2089–2098
Sohn D-R, Shin S-G, Park C-W, Kusaka M, Chiba K, Ishizaki T (1991) Metoprolol oxidation polymorphism in a Korean population: comparison with native Japanese and Chinese populations. Br J Clin Pharmacol 32: 504–507
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Hadidi, H.F., Irshaid, Y.M. & Rawashdeh, N.M. Metoprolol α-hydroxylation is a poor probe for debrizoquine oxidation (CYP2D6) polymorphism in Jordanians. Eur J Clin Pharmacol 47, 311–314 (1994). https://doi.org/10.1007/BF00191160
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191160