Abstract
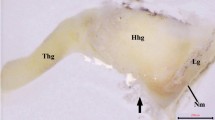
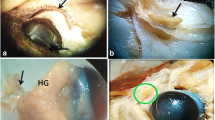
The orbital glands of the lizard Podarcis s. sicula are represented by the anterior and posterior lacrimal glands and the Harderian gland. The anlage of the Harderian gland appears on about the 22nd day of development in the form of a short tubule projecting from the conjunctival epithelium. This event is coincident with the appearance of the nictitating membrane. At this stage the mesenchymal cells surrounding the glandular blastema proliferate at a high rate and form a definite sac, later occupied by both the Harderian gland and the anterior lacrimal glands. At the 26th day of development, the glandular blastema forms acini at its distal end. The prospective glandular cells are not yet differentiated histologically. At the 36th day of development, differentiated serous glandular cells become visible. At the 41st day of development, the acini fill up the preformed mesenchymal sac. Only at this stage does the most medial part of the gland differentiate into mucous-secreting anterior lacrimal gland. At the same time, a small primordium of the posterior lacrimal gland can be seen in the posterior commissure of the eye. The appearance of junctional complexes between epithelial cells and mesenchymal cells in the early developmental stages supports the role of the mesenchyme in the differentiation of the glandular cells. Since the glandular anlage differentiates laterally into Harderian gland and medially into anterior lacrimal gland, spatial and temporal differences seem to exist in the inductive process. Furthermore, a concentration gradient of the inductive substance(s) may be envisaged, since an intermediate zone is present between the Harderian gland and the anterior lacrimal gland, consisting of mixed glandular cells containing both mucous and serous secretory granules.
Similar content being viewed by others
References
Bard J (1990) Morphogenesis: the cellular and molecular processes of developmental anatomy. Cambridge University Press, Cambridge
Bernfield MR, Banerjee SD, Koda JE, Rapraeger AC (1984) Remodelling of the basement membrane as a mechanism of morphogenetic tissue interaction. In: Trelstad RL (ed) The role of extracellular matrix in development. Liss, New York, pp 542–572
Burridge K, Fath K (1989) Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays 10:104–108
Chieffi Baccari G, Minucci S, Di Matteo L (1990a) Organogenesis of the Harderian gland in Rana esculenta and Bufo viridis. Boll Zool 57:221–224
Chieffi Baccari G, Minucci S, Di Matteo L, Chieffi G (1990b) Harderian gland and the lacrimal gland of the lizard, Podarcis s. sicula: histology, histochemistry and ultrastructure. Anat Rec 226:269–278
Chieffi Baccari G, Marmorino C, Minucci S, Di Matteo L, Varriale B, d'Istria M, Chieffi G (1992a) Mallory stain may indicate differential rates of RNA synthesis. I. A seasonal cycle in the Harderian gland of the green frog (Rana esculenta). Eur J Histochem 36:81–90
Chieffi Baccari G, Marmorino C, Minucci S, Di Matteo L, d'Istria M (1992b) Mallory stain may indicate differential rates of RNA synthesis. II. Comparative observations in vertebrate nuclei. Eur J Histochem 36:187–196
Dufaure JP, Hubert J (1961) Table de développement du lézard vivipare: Lacerta (zootoca) vivipara Jacquin. Arch Anat Microsc Morphol Exp 50:309–327
Michael MI, Khalil SH, Matta CA, Rizk TA (1988) Normal development of the ocular glands of the mouse. Folia Morphol 36:47–52
Payne AP (1994) The Harderian gland: a tercentennial review. J Anat 185:1–49
Sakai T (1981) The mammalian Harderian gland: morphology, histochemistry, function and phylogeny. Arch Histol Jpn 44:299–333
Shirama K, Hokano M (1991) Electron microscopic studies on the maturation of secretory cells in the mouse Harderian gland. Acta Anat 140:304–312
Shirama K, Kikuyama S, Takeo Y, Shimizu K, Mackawa K (1982) Development of Harderian gland during metamorphosis in anurans. Anat Rec 202:371–378
Wiedersheim R (1908) Der Bau des Menschen als Zeugnis für seine Vergangenheit. Laupp, Tübingen
Yurechenco MD, Schnittny JC (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chieffi Baccari, G., Di Matteo, L. & Minucci, S. Organogenesis of the orbital glands in the lizard Podarcis s. sicula: a histological, histochemical and ultrastructural study. Anat Embryol 192, 43–52 (1995). https://doi.org/10.1007/BF00186990
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186990