Abstract
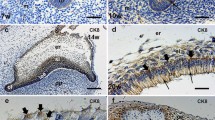
Distribution of gap junction protein in maxillary tooth germs of 1-day-old rats was examined by immunohistochemistry, using an affinity-purified antibody specific to residues 360–376 of rat connexin (CX) 43. In 1-day-old rats, the maxillary second molar formed the shape of the cusp, but neither dentine nor enamel was formed between the cells of the dental papilla and the inner enamel epithelium. In the tooth germ, CX 43 was expressed in the cells of the stratum intermedium and the inner enamel epithelium. Labelling in the stratum inter-medium was extensive and showed an increasing gradient from peripheral to cuspal regions. CX 43 detected in the inner enamel epithelium was at cell surfaces facing the interface between the dental papilla and the inner enamel epithelium. The cells of the dental papilla and the inner enamel epithelium began differentiation as odontoblasts and secretory ameloblasts respectively, in the cusps of the first molars, where predentine and dentine were formed but enamel matrix was not secreted. CX 43 was present in the stratum intermedium, inner enamel epithelium, preodontoblasts, odontoblasts and subodontoblasts. The incisors showed the most advanced stage of development, where the enamel matrix and calcified dentine were formed in the labial part of the teeth. The CX 43 epitope was seen in the stratum intermedium, inner enamel epithelium, preameloblasts, preodontoblasts, odontoblasts, and subodontoblasts. Immunolabelling was more extensive in the stratum intermedium and subodontoblasts than in preameloblasts, preodontoblasts, and odontoblasts. The immunolabelling in preameloblasts and preodontoblasts was accumulated at cell surfaces facing the predentine. Further, the labelling in preameloblasts and preodontoblasts disappeared or was reduced at the initiation of enamel matrix secretion and calcification of dentine matrix.
The present results suggest that gap junctional cell communication has important roles in tooth development. Further, the extensive CX 43 expression in the stratum intermedium and the subodontoblast layer suggests that gap junctions have an important role in amelogenesis and dentinogenesis.
Similar content being viewed by others
References
Akita H, Kobayashi Y, Kagayama M (1988) A histochemical study on lectin binding in immature enamel and secretory ameloblasts of rat incisors. Tohoku J Exp Med 155:139–149
Avery JK (1980) Pulp. In: Bhaskar SN (ed) Orban's oral histology and embryology. Mosby, London, pp 141–179
Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez JC (1991) Gap junctions: new tools, new answers, new questions. Neuron 6:305–320
Berkovitz BK, Maden M, Eriksson U (1993) The distribution of cellular retinoic acid-binding protein I during odontogenesis in the rat incisor. Arch Oral Biol 38:837–843
Beyer EC, Goodenough DA, Paul DL (1988) The connexins, a family of related gap junction proteins. Modern Cell Biol 7:175–176
Beyer EC, Paul DL, Goodenough DA (1990) Connexin family of gap junction protein. J Membr Biol 116:187–194
Bhussry BR (1968) Modification of the dental pulp organ during development and aging In: Sidney BF (ed) Biology of the dental pulp organ: a symposium, University of Alabama Press, Birmingham, pp 145–168
Bhussry BR (1980) Development and growth of teeth. In: Bhaskar SN (ed) Orban's oral histology and embryology, Mosby, London, pp 24–45
Chardin H, Septier D, Lecolle S, Goldberg M (1989) Effect of tunicamycin on glycogen accumulation in the stratum intermedium and odontoblasts of rat incisor. Cell Tissue Res 256:519–527
Dermietzel R, Hwang TK, Spray DS (1990) The gap junction family: structure, function and chemistry. Anat Embryol 182:517–528
Fromaget C, Aoumari AEI, Gros D (1992) Distribution pattern of connexin 43, a gap junctional protein, during the differentiation of mouse heart myocytes. Differentiation 51:9–20
Garant PR (1972) The demonstration of complex gap junctions between the cells of the enamel organ with lanthanum nitrate. J Ultrastruct Res 40:333–348
Gourdie RG, Green CR, Severs NJ, Thompson RP (1990) Three-dimensional reconstruction of gap junction arrangement in developing and adult rat hearts. Proc R Microsc Soc 1:417–420
Gourdie RG, Green CR, Severs NJ, Thompson RP (1992) Immunolabelling patters of gap junction connexins in the developing and mature rat heart. Anat Embryol 185:363–378
Guthrie SC, Gilula NB (1989) Gap junctional communication and development. Trends Neurosci 12:12–16
Inai T, Nagata K, Kurita T, Kurisu K (1992) Demonstration of amelogenin in the enamel-free cusps of rat molar tooth germs: immunofluorescent and immunoelectron microscopic studies. Anat Rec 233:588–596
Jepsen S, Schilts P, Strong DD, Scharla SH, Snead ML, Finkelman RD (1992) Transforming growth factor-betal mRNA in neonatal ovine molars visualized by in situ hybridization: potential role for the stratum intermedium. Arch Oral Biol 37:645–653
Jones SJ, Gray C, Sakamaki H, Arora M, Boyde A, Gourdie R, Green C (1993) The incidence and size of gap junctions between the bone cells in rat calvaria. Anat Embryol 187:343–352
Kempen MJA van, Gros D, Moorman AFM, Lamers WH (1991) Distribution of connexin 43 in the adult and developing rat heart. Circ Res 68:1638–1651
Kallenbach E (1977) Fine structure of secretory ameloblasts in the kitten. Am J Anat 148:479–511
Koike K, Watanabe H, Hiroi M, Tonosaki A (1993) Gap junction of stratum granulosum cells of mouse follicles: Immunohistochemistry and electron microscopy. J Electron Microsc 42:94–106
Lee S, Gilula NB, Warner AE (1987) Gap junctional communication and compaction during preimplantation stages of mouse development. Cell 51:851–860
Lennette DA (1978) An improved mounting medium for immunofluorescence microscopy. Am J Clin Pathol 69:647–648
Loewenstein WR (1981) Junctional intercellular communication: the cell to cell membrane channel. Physiol Rev 61:829–913
Mehta P, Bertram J, Loewenstein WR (1986) Growth inhibition of transformed cells correlates with junctional communication with normal cells. Cell 44:187–196
Orams HJ, Snibson KJ (1982) Ultrastructural localization and gradient of activity of alkaline phosphatase activity during rodent odontogenesis. Calcif Tissue Int 34:273–279
Ruangvoravat CP, Lo CW (1992) Connexin 43 expression in the mouse embryo: localization of transcripts within developmentally significant domains. Dev Dyn 194:261–281
Ruch JV, Lesot H, Karcher DV, Meyer JM, Olive M (1982) Facts and hypotheses concerning the control of odontoblast differentiation. Differentiation 21:7–12
Sasaki T, Garant PR (1986) Fate of annular gap junctions in the papillary cells of the enamel organ in the rat incisor. Cell Tissue Res 246:523–530
Sasaki T, Segawa K, Takiguchi R, Higashi S (1985) Formation of tight and gap junctions in the inner enamel epithelium and preameloblasts in human fetal tooth germs. Acta Anat Basel 121:223–229
Thesleff I, Barrack HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR (1981) Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev Biol 81:182–192
Thesleff I, Vainio S, Jalkanen M (1989) Cell-matrix interactions in tooth development. Int J Dev Biol 33:91–97
Warshawsky H (1978) A freeze-fracture study of the topographic relationship between the inner enamel-secretory ameloblasts in the rat incisor. Am J Anat 152:153–208
Weinstock M (1981) Gap junctions in the odontoblasts of the rat incisor teeth. Anat Rec 199:270A
Wise GE, Fan W (1989) Changes in the tartrate-resistent acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J Dent Res 68:150–156
Yancey SB, Biswal S, Revel JP (1992) Spatial and temporal patterns of distribution of the gap junction protein connexin 43 during mouse gastrulation and organogenesis. Development 114:203–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kagayama, M., Akita, H. & Sasano, Y. Immunohistochemical localization of connexin 43 in the developing tooth germ of rat. Anat Embryol 191, 561–568 (1995). https://doi.org/10.1007/BF00186744
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186744