Summary
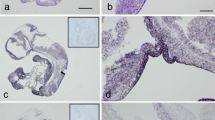
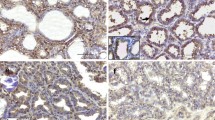
Pleiotrophin (PTN), also known as HBGAM, belongs to an emerging cytokine family unrelated to other growth factors. We report here the first comprehensive study using in situ hybridization on the cellular distribution of this new heparin-binding growth factor mRNA in rat tissues. PTN mRNA was developmentally expressed in many — but not all — neuroectodermal and mesodermal lineages, whilst no PTN mRNA was detected in endoderm, ectoderm and trophoblast. PTN mRNA was found in the nervous system throughout development, with a post-natal peak of expression. In the adult nervous system, significant expression persisted in hippocampal CA1 pyramidal neurons and in cortical neurons, but also in different non-neuronal cells types in various locations (olfactory nerve, cerebellar astrocytes, pituicytes, Schwann cells surrounding the neurons in sensory ganglia). PTN mRNA was also found during development in the mesenchyme of lung, gut, kidney and reproductive tract, in bone and cartilage progenitors, in dental pulp, in myoblasts, and in several other sites. Expression was differently regulated in each location, but usually faded around birth. In the adult, PTN mRNA was still present in the meninges, the iris, the Leydig cells of the testis and in the uterus. PTN mRNA was also strongly expressed in the basal layers of the tongue epithelium, which is the only epithelium and ectodermal derivative to express PTN mRNA, and this only after birth. PTN is known to be a growth factor for perinatal brain neurons and a mitogen for fibroblasts in vitro. Recently, trophic effects on epithelial cells and a role as a tumour growth factor have been reported. The mechanisms of regulation and the functions of PTN are however still uncertain. Its expression pattern during development suggests important roles in growth and differentiation. Moreover, the presence of PTN mRNA in several adult tissues and the up-regulation of PTN mRNA expression in the gravid uterus indicate that PTN also has physiological functions during adulthood.
Similar content being viewed by others
References
Baetge G, Pintar JE, Gershon MD (1990) Transient catecholamin-ergic (TC) cells in the bowel of the fetal rat: precursors of noncatecholaminergic enteric neurons. Dev Biol 141:353–380
Beaulieu JF, Vachon PH, Chartrand S (1991) Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol 183:363–369
Christie GA (1964) Developmental stages in somite and post-somite rat embryos, based on external appearance and including some features of the macroscopic development of the oral cavity. J Morphol 114:263–286
Kadomatsu K, Tomomura M, Muramatsu T (1988) cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun 151:1312–1318
Karns LR, Ng S-C, Freeman JA, Fishman MC (1987) Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science 236:597–600
Kedinger M, Simon-Assmann P, Bouzigues F, Arnold C, Alex-andre E, Haffen K (1990) Smooth muscle actin expression during rat gut development and induction in fetal skin fibroblastic cells associated with intestinal embryonic epithelium. Differentiation 43:87–97
Kuo M-D, Huang SS, Huang JS (1992) Characterization of hepar-in-binding growth-associated factor on NIH 3T3 cells. Biochem Biophys Res Commun 182:188–194
Landry CF, Ivy GO, Brown IR (1990) Developmental expression of the glial fibrillary acidic protein mRNA in the rat brain analyzed by in situ hybridization. J Neurosci Res 25:194–203
Levi-Montalcini R, Bookes B (1960) Proc Natl Acad Sci USA 46:384–91
Li Y-S, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrant J, Deuel TF (1990) Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science 250:1690–1694
Lyons GE, Ontell M, Cox R, Sassoon D, Buckingham M (1990) The expression of myosin genes in developing skeletal muscle in the mouse embryo. J Cell Biol 111:1465–1476
Merenmies J, Rauvala H (1990) Molecular cloning of the 18-kDa growth-associated protein of developing brain. J Biol Chem 265(28):16721–16724
Milner PG, Li Y-S, Hoffman RM, Kodner CM, Siegel NR, Deuel TF (1989) A novel 17 kD heparin-binding growth factor (HBGF-8) in bovine uterus: purification and N-terminal amin-oacid sequence. Biochem Biophys Res Commun 165(3):1096–1103
Nakanishi K, Kitamura T, Okuda M, Mazaki T, Watanabe S, Miyoshi N, Fujita S, Fukuda M (1990) Analysis of GFA-pro-tein mRNA expression in developing bovine brain by in situ hybridization and Northern blot hybridization. Basic Appl Histochem 34(2):101–110
Perkins AS, Mercer JA, Jenkins NA, Copeland NG (1991) Patterns of EVI-1 expression in embryonic and adult tissues suggest that EVI-1 plays an important regulatory role in mouse development. Development 111:479–487
Raulais D, Lagente-Chevalier O, Guettet C, Duprez D, Courtois Y, Vigny M (1991) A new heparin-binding protein regulated by retinoic acid from chicken embryo. Biochem Biophys Res Commun 174(2):708–715
Rauvala H (1989) An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J 8(10):2933–2941
Rugh R (1990) The mouse. Its reproduction and development. Oxford University Press
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press
Sawtell NM and Lessard JL (1989) Cellular distribution of smooth muscle actins during mammalian embryogenesis: Expression of the α-Vascular but not the γ-Enteric isoform in differenciat-ing striated myocytes. J Cell Biol 109:2929–2937
Schiffmann SN, Vanderhaeghen J-J (1991) Distribution of cells containing mRNA encoding cholecystokinin in the rat central nervous system. J Comp Neurol 304:219–233
Scott J, Selby M, Urdea M, Quiroga M, Bell GI, Rutter WJ (1983) Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature 302:538–540
Sharkey KA, Coggins PJ, Tetzlaff W, Zwiers H, Bisby MA, Dav-ison JS (1990) Distribution of growth-associated protein B-50 (GAP-43) in the mammalian enteric nervous system. Neuroscience 38:13–20
Tabin CJ (1991) Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell 66:199–217
Tezuka KI, Takeshita S, Hakeda Y, Kumegawa M, Kikuno R, Hashimoto-Gotoh T (1990) Isolation of mouse and human cDNA clones encoding a protein expressed specifically in osteoblasts and brain tissues. Biochem Biophys Res Commun 173:246–251
Theiler K (1989) The house mouse. Atlas of embryonic development. Springer, New York
Tomomura M, Kadomatsu K, Nakamoto M, Muramatsu H, Kon-doh H, Imagawa K, Muramatsu T (1990) Biochem Biophys Res Comm 171:603–609
Wellstein A, Fang W, Khatri A, Lu Y, Swain SS, Dickson RB, Sassel J, Riegel AT, Lippman ME (1992) A heparin-binding growth factor secreted from breast cancer cells homologous to a developmentally regulated cytokine. J Biol Chem 267(4):2582–2587
Wewetzer K, Rauvala H, Unsicker K (1991) HB-GAM immunoreactivity in the developing rat cerebellum. Soc Neurosci Abstr 17:556/224.5
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vanderwinden, J.M., Mailleux, P., Schiffmann, S.N. et al. Cellular distribution of the new growth factor Pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat Embryol 186, 387–406 (1992). https://doi.org/10.1007/BF00185989
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185989