Abstract
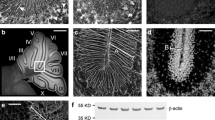
It has been considered that cortical malformation in the brain of the reeler mutant mouse is due to a defect in the process of neuroblast migration during development. We examined the process of Purkinje cell migration in the cerebellar primordium of the reeler mutant immunohistochemically and electron-microscopically, employing a specific marker for Purkinje cells and markers for radial glia. To facilitate the recognition of the homozygote of the reeler mutation (r1) at the embryonic stage, we introduced the chromosome carrying the autosomal semi-dominant mutation, hammer-toe (Hm), by crossbreeding and backcross into the heterozygote of the reeler mutation, which is an autosomal recessive and located on the homologous chromosome. Using this double heterozygous strain (+/rl-Hm/+), the homozygote of rl can be selected from littermates by the normal appearance of the feet. Both the heterozygous rl embryos and non-carriers harbor the Hm locus and show the Hm phenotype as a deformity of the feet that can be recognized from the 15th day of gestation. In the cerebellar primordium of control mice, Purkinje cells migrated radially from the ventricular zone towards the cortex. In contrast, most of the migratory Purkinje cells remained in the intermediate zone, and their migration towards the cortex was obstructed in the cerebellum of the reeler mutant. A disorganized arrangement of both the processes and cell bodies of the radial glia was demonstrated in the cerebellar primordium of the reeler by labeling them with the antibody against tenascin, a neuron-glial adhesion molecule, and the monoclonal antibody 1D11, a marker for immature astroglia. Electron-microscopic observations revealed apposition of the migratory cells to the radially oriented glial processes in the intermediate zone of the control cerebellum. In contrast, the apposition of leading processes of the migratory neuroblasts to disorganized processes of the radial glia was observed in the intermediate zone of the reeler cerebellum. These findings suggest that the obstructed migration and disordered cortical alignment of Purkinje cells in the reeller cerebellum is due to dysgenesis and abnormal development of radial glia, resulting in disturbance of contact guidance in the process of Purkinje cell migration.
Similar content being viewed by others
References
Bignami A, Dahl D (1974) The development of Bergmann glia in mutant mice with cerebellar malformations: reeler, staggerer and weaver. Immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol 155:219–230
Caviness VS Jr, Rakic P (1978) Mechanisms of cortical development: a view from mutations in mice. Ann Rev Neurosci 1:297–326
Caviness VS Jr, Crandall JE, Edwards MA (1988) The reeler malformation — implications for neocortical histogenesis. In: Peters A, Jones EG (eds) Cerebral cortex, vol 7, Development and maturation of cerebral cortex. Plenum Press, New York, pp 59–89
Crossin KL, Prieto AL, Hoffman S, Jones FS, Friedlander DR (1990) Expression of adhesion molecules and the establishment of boundaries during embryonic and neural development. Exp Neurol 109:6–18
Davisson MT, Roderick TH (1989) Linkage map. In: Lyon MF, Searle AG (eds) Genetic variants and strains of the laboratory mouse, 2nd edn. Oxford University Press, Oxford, p 420
Del Cerro M, Swarz JR (1976) Prenatal development of Bergmann glial fibres in rodent cerebellum. J Neurocytol 5:669–676
Gadisseux J-F, Evrard P (1985) Glial-neuronal relationship in the developing central nervous system — a histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Dev Neurosci 7:2–32
Godfraind C, Schachner M, Goffinet AM (1988) Immunohistological localization of cell adhesion molecules L1, J1, N-CAM and their common carbohydrate L2 in the embryonic cortex of normal and reeler mice. Dev Brain Res 42:99–111
Goffinet AM (1979) An early developmental defect in the cerebral cortex of the reeler mouse — a morphological study leading to a hypothesis concerning the action of the mutant gene. Anat Embryol 157:205–216
Goffinet AM (1983) The embryonic development of the cerebellum in normal and reeler mutant mice. Anat Embryol 168:73–86
Green MC (1964) Mouse News Lett 31:27
Green MC (1989a) Catalog of mutant genes and polymorphic loci. In: Lyon MF, Searle AG (eds) Genetic variants and strains of the laboratory mouse, 2nd edn. Oxford University Press, Oxford, p 163
Green MC (1989b) Catalog of mutant genes and polymorphic loci. In: Lyon MF, Searle AG (eds) Genetic variants and strains of the laboratory mouse, 2nd edn. Oxford University Press, Oxford, pp 311–312
Hatten ME (1985) Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol 100:384–396
Hatten ME, Mason CA (1990) Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia 46:907–916
Oda S, Hoshino K (1989) Recombination value of reeler, rl and hammer-toe, Hm gene loci on chromosome 5 in the mouse (in Japanese). Ann Res Inst Environ Med Nagoya Univ 40:266–267
Ono K. Yanagihara M, Mizukawa K, Yuasa S, Kawamura K (1989) Monoclonal antibody that binds to both the prenatal and postnatal astroglia in rodent cerebellum. Dev Brain Res 50:154–159
Pinto-Lord MC, Evrard P, Caviness VS Jr. (1982) Obstructed neuronal migration along radial glial fibers in the neocortex of the reeler mouse: a Golgi-EM analysis. Dev Brain Res 4:379–393
Rakic P (1990) Principles of neural cell migration. Experientia 46:882–891
Shur BD (1982) Galactosyltransferase defects in reeler mouse brain. J Neurochem 39:201–209
Steindler DA, O'Brien TF, Laywell E, Harrington K, Faissner A, Schachner M (1990) Boundaries during normal and abnormal brain development: in vivo and in vitro studies of glia and glycoconjugates. Exp Neurol 109:35–56
Weller A, Beck S, Ekblom P (1991) Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol 112:355–362
Yamakuni T, Usui H, Iwanaga T, Kondo H, Odani S, Takahasi Y (1984) Isolation and immunohistochemical localization of a cerebellar protein. Neurosci Lett 45:235–240
Yuasa S, Kawamura K, Ono K, Yamakuni T, Takahashi Y (1991) Development and migration of Purkinje cells in the mouse cerebellar primordium. Anat Embryol 184:195–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yuasa, S., Kitoh, J., Oda, Si. et al. Obstructed migration of Purkinje cells in the developing cerebellum of the reeler mutant mouse. Anat Embryol 188, 317–329 (1993). https://doi.org/10.1007/BF00185941
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185941