Summary
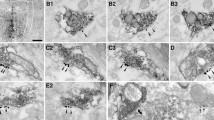
We examined the synaptic organization of ascending projections from the pars ventralis of the dorsal tegmental nucleus of Gudden (TDV) and the laterodorsal tegmental nucleus to the lateral mammillary nucleus (LM). The LM neuropil consists of terminals containing pleomorphic synaptic vesicles and forming symmetric synaptic contact, and terminals containing round synaptic vesicles and forming asymmetric synaptic contact. They make up 63% and 37%, respectively, of all axodendritic terminals. All axosomatic terminals contain pleomorphic vesicles and make symmetric contact. Following injection of WGA-HRP into the TDV, many anterogradely labeled terminals and retrogradely labeled cells are found in the LM. Labeled terminals contact mainly proximal (more than 2 μm diameter) and intermediate (1–2 μm diameter) dendrites. Serial ultrathin sections of the LM show that 55% of axosomatic terminals are labeled anterogradely. Following injection of WGA-HRP into the laterodorsal tegmental nucleus, many anterogradely labeled terminals are found in the LM, but no retrogradely labeled cells are present. Labeled terminals contact mainly distal (less than 1 μm diameter) and intermediate dendrites as well as somata. In the LM neurons, 46% of axosomatic terminals are labeled anterogradely. All labeled terminals from these nuclei contain pleomorphic vesicles and make symmetric synaptic contact. These results indicate that almost all axosomatic terminals come from the TDV and the laterodorsal tegmental nucleus, which send inhibitory inputs to the lateral mammillary nucleus.
Similar content being viewed by others
References
Aggleton LP, Mishkin M (1985) Mamillary-body lesions and visual recognition in monkeys. Exp Brain Res 58:190–197
Allen GV, Hopkins DA (1988) Mamillary body in the rat: a cytoarchitectonic, Golgi, and ultrastructural study. J Comp Neurol 275:39–64
Allen GV, Hopkins DA (1989) Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol 286:311–336
Allen GV, Hopkins DA (1990) Topography and synaptology of mamillary body projections to the mesencephalon and pons in the rat. J Comp Neurol 301:214–231
Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD (1983) Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol 216:53–68
Berk ML, Finkelstein JA (1981) Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience 6:1601–1624
Bobillier P, Petitjean F, Salvert D, Ligier M, Seguin S (1975) Differential projections of the nucleus raphé dorsalis and nucleus raphé centralis as revealed by autoradiography. Brain Res 85:205–210
Briggs TL, Kaelber WW (1971) Efferent fiber connections of the dorsal and deep tegmental nuclei of Gudden. An experimental study in the cat. Brain Res 29:17–29
Cruce JAF (1977) An autoradiographic study of the descending connections of the mammillary nuclei of the rat. J Comp Neurol 176:631–644
Finley JCW, Maderdrut JL, Petrusz P (1981) The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol 198:541–555
Frotscher M, Léránth C (1985) Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239:237–246
Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJH (1986) Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol 249:65–102
Hallanger AE, Wainer BH (1988) Ultrastructure of ChAT-immunoreactive synaptic terminals in the thalamic reticular nucleus of the rat. J Comp Neurol 278:486–497
Harlan RE, Shivers BD, Romano GJ, Howells RD, Pfaff D (1987) Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol 258:159–184
Harlan RE, Garcia MM, Krause JE (1989) Cellular localization of substance P- and neurokinin A-encoding preprotachykinin mRNA in the female rat brain. J Comp Neurol 287:179–212
Hattori T, McGeer EG, Singh VK, McGeer PL (1977) Cholinergic synapse of the interpeduncular nucleus. Exp Neurol 55:666–679
Hayakawa T, Zyo K (1982) Organization of the habenulo-interpeduncular connections in cats: a horseradish peroxidase study. Brain Res 240:3–11
Hayakawa T, Zyo K (1984) Comparative anatomical study of the tegmentomammillary projections in some mammals: a horse-radish peroxidase study. Brain Res 300:335–349
Hayakawa T, Zyo K (1985) Afferent connections of Gudden's tegmental nuclei in the rabbit. J Comp Neurol 235:169–181
Hayakawa T, Zyo K (1986) Subcortical afferents to the nucleus reticularis tegmenti pontis in the rabbit: a retrograde horseradish peroxidase study. Okajimas Folia Anat Jpn 63:159–178
Hayakawa T, Zyo K (1989) Retrograde double-labeling study of the mammillothalamic and the mammillotegmental projections in the rat. J Comp Neurol 284:1–11
Hayakawa T, Zyo K (1990a) Ultrastructure of the mammillotegmental projections to the ventral tegmental nucleus of Gudden in the rat. J Comp Neurol 293:466–475
Hayakawa T, Zyo K (1990b) Fine structure of the lateral mammillary projections to the dorsal tegmental nucleus of Gudden in the rat. J Comp Neurol 298:224–236
Hayakawa T, Zyo K (1991) Quantitative and ultrastructural study of ascending projections to the medial mammillary nucleus in the rat. Anat Embryol 184:611–622
Hayakawa T, Seki M, Zyo K (1981) Studies on the efferent projections of the interpeduncular complex in cats. Okajimas Folia Anat Jpn 58:1–16
Henry MA, Westrum LE, Johnson LR (1985) Ultrastructure of transganglionic HRP transport in cat trigeminal system. Brain Res 334:255–266
Herkenham M, Nauta WJH (1979) Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187:19–48
Holmes EJ, Butters N, Jacobson S, Stein BM (1983a) An examination of the effects of mammillary lesions on reversal learning sets in monkeys. Physiol Psychol 11:159–165
Holmes EJ, Jacobson S, Stein BM, Butters N (1983b) Ablations of mammillary nuclei in monkeys: effects on postoperative memory. Exp Neurol 81:97–113
Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE (1983) Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res 266:97–119
Isaacson LG, Tanaka D Jr (1988) Cholinergic innervation of canine thalamostriatal projection neurons: an ultrastructural study combining choline acetyltransferase immunocytochemistry and WGA-HRP retrograde labeling. J Comp Neurol 277:529–540
Itaya SK (1987) Anterograde transsynaptic transport of WGA-HRP in rat olfactory pathways. Brain Res 409:205–214
Kaneko T, Itoh K, Shigemoto R, Mizuno N (1989) Glutaminase-like immunoreactivity in the lower brainstem and cerebellum of the adult rat. Neuroscience 32:79–98
Khachaturian H, Lewis ME, Watson SJ (1983) Enkephalin system in diencephalon and brainstem of the rat. J Comp Neurol 220:310–320
Liu R, Chang L, Wickern G (1984) The dorsal tegmental nucleus: an axoplasmic transport study. Brain Res 310:123–132
Meibach RC, Siegel A (1977) Efferent connections of the hippocampal formation in the rat. Brain Res 124: 197–224
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Morest DK (1961) Connexions of the dorsal tegmental nucleus in rat and rabbit. J Anat 95:229–249
Mugnaini E, Oertel WH (1985) An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 4. GABA and Neuropeptides in the CNS, Part I. Elsevier, Amsterdam, pp 406–608
Niimi K, Koizuka M, Kawamura S, Abe K (1972) Efferent projections of the mamillary body in the cat. Okajimas Folia Anat Jpn 49:129–156
Olucha F, Martinez-Garcia F, López-Garcia C (1985) A new stabilizing agent for tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP). J Neurosci Methods 13:131–138
Peters A, Palay SL, Webster HdeF (1991) The fine structure of the nervous system. Neurons and their supporting cells. 3rd edn. Oxford University Press, Oxford New York, pp 138–211
Petrovicky P (1973) Note on the connections of Gudden's tegmental nuclei. 1. Efferent ascending connections in the mamillary peduncle. Acta Anat 86:165–190
Petrovický P (1985) Gudden's tegmental nuclei and their connections to the hypothalamus and the reticular formation. 1. An experimental study using retrograde labelling with HRP or iron-dextran in the rat. J Hirnforsch 26:531–537
Petrusz P, Merchenthaler I, Maderdrut JL (1985) Distribution of enkephalin-containing neurons in the central nervous system. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 4. GABA and Neuropeptides in the CNS, Part I. Elsevier, Amsterdam, pp 273–334
Porter JD, Guthrie BL, Sparks DL (1985) Selective retrograde transneuronal transport of wheat germ agglutinin-conjugated horseradish peroxidase in the oculomotor system. Exp Brain Res 57:411–416
Ruggiero DA, Giuliano R, Anwar M, Storentta R, Reis DJ (1990) Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol 292:1–53
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–392
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topograph- ical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242
Shibata H (1987) Ascending projections to the mammillary nuclei in the rat: a study using retrograde and anterograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. J Comp Neurol 264:205–215
Shibata H (1989) Descending projections to the mammillary nuclei in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol 285:436–452
Shibata K, Furukawa T (1988) The mammillary body, a potential site of action of neurotensin in passive avoidance behavior in rats. Brain Res 443:117–124
Shibata H, Suzuki T (1984) Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: a retrograde horseradish peroxidase study. Brain Res 296:345- 349
Shibata K, Kataoka Y, Yamashita K, Ueki S (1986) An important role of the central amygdaloid nucleus and mammillary body in the mediation of conflict behavior in rats. Brain Res 372:159–162
Sutin E, Jacobowitz DM (1988) Immunocytochemical localization of peptides and other neurochemicals in the rat laterodorsal tegmental nucleus and adjacent area. J Comp Neurol 270:243–270
Swanson LW, Cowan WM (1977) An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84
Takeuchi Y, Allen GV, Hopkins DA (1985) Transnuclear transport and axon collateral projections of the mamillary nuclei in the rat. Brain Res Bull 14:453–468
Terreberry RR, Neafsey EJ (1987) The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull 19:639–649
Uchizono K (1965) Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207:642–643
Uchizono K (1967) Synaptic organization of the Purkinje cells in the cerebellum of the cat. Exp Brain Res 4:97–113
Veazey RB, Amaral DG, Cowan WM (1982a) The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). I. Cytoarchitectonic organization. J Comp Neurol 207:114–134
Veazey RB, Amaral DG, Cowan WM (1982b) The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). II. Efferent connections. J Comp Neurol 297:135–156
Vertes RP (1988) Brainstem afferents to the basal forebrain in the rat. Neuroscience 24:907–935
Vertes RP, Martin GF (1988) Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 275:511–541
Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD (1984) Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res 308:69–76
Williams RG, Dockray GJ (1983) Distribution of enkephalin-related peptides in rat brain: immunohistochemical studies using antisera to Met-enkephalin and Met-enkephalin Arg6Phe7. Neuroscience 9:563–586
Woolf NJ, Harrison JB, Buchwald JS (1990) Cholinergic neurons of the feline pontomesencephalon. II. Ascending anatomical projections. Brain Res 520:55–72
Yamazoe M, Shiosaka S, Emson PC, Tohyama M (1985) Distribution of neuropeptide Y in the lower brainstem: an immunohistochemical analysis. Brain Res 335:109–120
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayakawa, T., Zyo, K. Ultrastructural study of ascending projections to the lateral mammillary nucleus of the rat. Anat Embryol 185, 547–557 (1992). https://doi.org/10.1007/BF00185614
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185614