Abstract
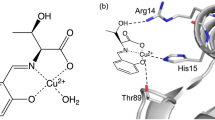
The activity profile of the CU2Zn2HSOD Ile-137 mutant has a pKa of 9.6, i. e. one unit lower than the wild type (WT). This property has allowed us to investigate the inactive high pH form of the enzyme before denaturation occurs. The electronic and EPR spectra do not change with the above pKa. The 1H NMR spectrum of the CU2CO2-analog reveals slight decreases in the hyperfine shifts of the protons of His-48 at high pH, which are consistent with a water molecule becoming closer to the copper ion, as detected through water 1H T 1 −1 NMR measurements. The affinity of azide at high pH is lower than at low pH, though still sizeable. The WT follows the same pattern up to pH ≅ pKa. It appears that the drop in activity is not related to any major change involving the metal coordination sphere, but is related to changes in the electrostatic potential due to the deprotonation process.
Similar content being viewed by others
References
Argese E, Rigo A, Viglino P, Orsega E, Marmocchi F, Cocco D, Rotilio G (1984) A study of the pH dependence of the activity of porcine Cu, Zn superoxide dismutase. Biochim Biophys Acta 787:205–207
Banci L, Bertini I, Luchinat C, Scozzafava A (1987) Nuclear relaxation in the magnetic coupled system CU2Co2SOD. Histidine-44 is detached upon anion binding. J Am Chem Soc 109:2328–2334
Banci L, Bertini I, Luchinat C, Hallewell RA (1988) The exploration of the active-site cavity of copper-zinc superoxide dismutase. Ann NY Acad Sci 542:37–52
Banci L, Bertini I, Hallewell RA, Luchinat C, Viezzoli MS (1989a) The water in the cavity of copper zinc superoxide dismutase. A water 1H NMRD study. Eur J Biochem 184:125–129
Banci L, Bertini I, Luchinat C, Piccioli M, Scozzafava A, Turano P (1989b) 1H NOE studies on copper (II)2cobalt (II)2 superoxide dismutase. Inorg Chem 28:4650–4656
Banci L, Bertini I, Luchinat C, Scozzafava A, Turano P (1989c) The binding of fluoride to copper zinc superoxide dismutase. Inorg Chem 28:2377–2381
Banci L, Bencini A, Bertini I, Luchinat C, Viezzoli MS (1990a) The angular overlap analysis of the spectroscopic parameters of copper-zinc SOD and its mutants. Gazz Chim Ital (in press)
Banci L, Bencini A, Bertini 1, Luchinat C, Piccioli M (1990b) 1H NOE and ligand field studies of copper-cobalt superoxide dismutase with anions. Inorg Chem (in press)
Beem KM, Rich WE, Rajagopalan KV (1974) Total reconstitution of copper-zinc superoxide dismutase. J Biol Chem 249:7298–7305
Bertini I, Luchinat C (1986) NMR of paramagnetic molecules in biological systems. Cummings, Boston, Mass
Bertini I, Borghi E, Luchinat C, Scozzafava A (1981 a) 2. Binding sites of anions in superoxide dismutase. J Am Chem Soc 103:7779–7783
Bertini I, Luchinat C, Messori L (1981 b) A water 17O NMR study of the pH dependent properties of superoxide dismutase. Biochim Biophys Res Commun 101:57–583
Bertini I, Briganti F, Luchinat C, Mancini M, Spina G (1985 a) The electron-nucleus coupling in slow rotating systems. 2. The effect of g anysotropy and hyperfine coupling when S = 1/2 and I = 3/2. J Magn Reson 63:41–55
Bertini I, Lanini G, Luchinat C, Messori L, Monnanni R, Scozzafava A (1985 b) An investigation of Cu2Co2SOD and its anion derivatives. 1H NMR and electronic spectra. J Am Chem Soc 107:4391–4396
Bertini I, Banci L, Luchinat C (1988) NMR of paramagnetic systems. In: Lawrence Que Jr (ed) Metal cluster in proteins. Am Chem Soc Symp Set, 372, Chap 4, pp 70–84
Bertini I, Banci L, Luchinat C, Bielski BHJ, Cabelli DE, Mullenbach GT, Hallewell RA (1989) An investigation of a human erythrocyte SOD modified at position 137. J Am Chem Soc 111:714–719
Boden N, Holmes MC, Knowles PE (1979) Properties of the cupro sites in bovine superoxide dismutase studied by nuclear-relaxation measurements. Biochem J 177:303–309
Briggs RG, Fee JA (1978) Further characterization of human erythrocyte superoxide dismutase. Biochim Biophys Acta 537:86–99
Desideri A, Comin F, Morpurgo L, Cocco D, Calabrese L, Mondovi B, Maret W, Rotilio G (1981) X-ray absorption edge spectroscopy of Co(II)-binding sites of copper- and zinc-containing proteins. Biochim Biophys Acta 661:312–315
Fee JA (1973) Studies on the reconstitution of bovine erythrocyte superoxide dismutase. IV. Preparation and some properties of the enzyme in which Co(II) is substituted for Zn(II). J Biol Chem 248:4229–4234
Fee JA (1981) Is superoxide toxic and are superoxide dismutase essential for aerobic life? In: Rodgers MAJ, Power EL (eds) Oxygen and oxy-radicals in chemistry and biology. Academic Press, New York, pp 205–239
Fee JA, Gaber bP (1972) Anion binding to bovine erythrocyte superoxide dismutase. J Biol Chem 247:60–65
Fridovich I (1987) Superoxide dismutases. Adv Enzymol Relat Ares Mol Biol 58:61–97
Getzoff ED, Tainer JA, Weiner PK, Kollmann PA, Richardson JS, Richardson DC (1983) Electrostatic interaction between superoxide and copper, zinc superoxide dismutase. Nature 306:287–290
Hallewell R, Masiarz F, Najarian R, Puma J, Quiroga M, Randolph A, Sanchez-Pescador R, Scandella C, Smith B, Steimer K, Muellenbach G (1985) Human Cu/Zn superoxide dismutase cDNA: isolation of clones synthesizing high levels of active or inactive enzyme from an expression library. Nucleic Acids Res 13:2017–2034
Hallewell RA, Mills R, Tekamp-Olson P, Blacher R, Rosenberg S, Otting F, Masiarz FR, Scandella CF (1987) Amino terminal acetylation of authentic human Cu, Zn superoxide dismutase product in yeast. Biotechnology 5:363–366
Inubushi T, Becker ED (1983) Efficient detection of paramagnetically shifted NMR resonances by optimizing the WEFT pulse sequence. J Magn Res 51:128–133
Klug D, Rabani J, Fridovich I (1972) A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem 247:4839–4842
McCord MW, Fridovich I (1969) Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Ming L-J, Banci L, Luchinat C, Bertini I, Valentine JS (1988) NMR study of cobalt (II)-substituted yeast and human copper-zinc superoxide dismutase. Inorg Chem 27:728–733
Morgenstern-Badarau I, Cocco D, Desideri A, Rotilio G, Jordanov J, Dupré N (1986) Magnetic susceptibility studies of the native cupro-zinc superoxide dismutase and its cobalt-substituted derivatives. Antiferromagnetic coupling in the imidazolato bridged copper (II)-cobalt (II) pair. J Am Chem Soc 108:300–302
Pantoliano MW, Valentine JS, Mammone RJ, Scholler DM (1979) pH dependence of metal ion binding to the native zinc site of bovine erythrocuprein (superoxide dismutase) J Am Chem Soc 101:6354–7456
Pantoliano MW, Valentine JS, Nafie LA (1982) Spectroscopic studies of copper(II) bound at the native copper site or substitued at the native zinc site of bovien erythrocuprein (superoxide dismutase). J Am Chem Soc 104:6310–6317
Rotilio G, Finazzi Agró A, Calabrese L, Bossa F, Guerrieri P, Mondovi B (1972) Studies of the metal sites of copper proteins. Ligands of copper in hemocuprein. Biochemistry 10:616–621
Rotilio G, Morpurgo L, Giovagnoli C, Calabrese L, Mondovi B (1972) Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry 11:2187–2192
Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC (1982) Determination and analysis of the 2 Å structure of copper, zinc superoxide dismutase. J Mol Biol 160:181–217
Tainer JA, Getzoff ED, Richardson JS, Richardson DC (1983) Structure and mechanism of copper, zinc superoxide dismutase. Nature 306:284–287
Terenzi M, Rigo A, Franconi C, Mondovi B, Calabrese L, Rotilio G (1974) pH dependence of the nuclear magnetic relaxation rate of solvent water protons in solutions of bovine superoxide dismutase. Biochim Biophys Acta 351:230–236
Valentine JS, Pantoliano MW (1981) Protein-metal ion interactions in cuprozinc protein (superoxide dismutase). In: Spiro TG (ed) Metal ions in biology, vol 3. Wiley, New York, pp 291–358
Vold RL, Waugh JS, Klein MP, Phelps DE (1968) Measurement of spin relaxation in complex systems. J Chem Phys 48:3831–3832
Author information
Authors and Affiliations
Additional information
Offprint requests to: I. Bertini
Rights and permissions
About this article
Cite this article
Banci, L., Bertini, I. & Turano, P. An investigation of Cu2Zn2 superoxide dismutase and its Ile-137 mutant at high pH. Eur Biophys J 19, 141–146 (1991). https://doi.org/10.1007/BF00185454
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185454