Abstract
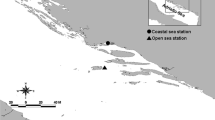
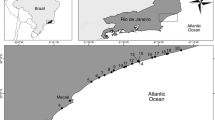
The depth-dependent, seasonal, and diel variability of virus numbers, dissolved DNA (D-DNA), and other microbial parameters was investigated in the northern Adriatic Sea. During periods of water stratification, we found higher virus abundances and virus/bacterium ratios (VBRs) as well as a larger variability of D-DNA concentrations at the thermocline, probably as a result of higher microbial biomass. At the two investigated stations, virus densities were highest in summer and autumn (up to 9.5 × 1010 1−1) and lowest in winter (< 109 1−1); D-DNA concentrations were highest in summer and lowest in winter. The VBR as well as an estimated proportion of viral DNA on total D-DNA showed a strong seasonal variability. VBR averaged 15.0 (range, 0.9–89.1), and the percentage of viral DNA in total D-DNA averaged 18.3% (range, 0.1–96.1%). An estimation of the percentage of bacteria lysed by viruses, based on 2-h sample intervals in situ, ranged from 39.6 to 212.2% d−1 in 5 m and from 19.9 to 157.2% d−1 in 22 m. The estimated contribution of virus-mediated bacterial DNA release to the D-DNA pool ranged from 32.9 to 161% d−1 in 5 m and from 10.3 to 74.2% d−1 in 22 m. Multiple regression analysis and the diel dynamics of microbial parameters indicate that viral lysis occasionally could be more important in regulating bacterial abundances than grazing by heterotrophic nanoflagellates.
Similar content being viewed by others
References
Baines SB, Pace ML (1991) The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater system. Limnol Oceanogr 36:1078–1090
Beebee TJC (1991) Analysis, purification and quantification of extracellular DNA from aquatic environments. Freshwater Biol 25:525–532
Bergh Ø, Børsheim KY, Bratbak G, Heldal M (1989) High abundance of viruses found in aquatic environments. Nature 340:467–468
Boehme J, Frischer ME, Jiang SC, Kellogg CA, Pichard S, Rose JB, Steinway C, Paul JH (1993) Viruses, bacterioplankton, and phytoplankton in the southeastern Gulf of Mexico: distribution and contribution to oceanic DNA pools. Mar Ecol Prog Ser 91:1–10
Børsheim KY, Bratbak G, Heldal M (1990) Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol 56:352–356
Bratbak G, Egge JK, Heldal M (1993) Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar Ecol Prog Ser 93:39–48
Bratbak G, Heldal M, Norland S, Thingstad TF (1990) Viruses as partners in spring bloom microbiol trophodynamics. Appl Environ Microbiol 56:1400–1405
Bratbak G, Heldal M, Thingstad TF, Riemann B, Haslund OH (1992) Incorporation of viruses in the budget of microbial C-transfer. A first approach. Mar Ecol Prog Ser 83:273–280
Caron DA (1983) Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol 46:491–498
Cho BC, Azam F (1988) Heterotrophic bacterioplankton production measurement by the tritiated thymidine incorporation method. Arch Hydrobiol Beih 31:153–162
Cochlan WP, Wikner J, Steward GF, Smith DC, Azam F (1993) Spatial distribution of viruses, bacteria and chlorophyll a in neritic, oceanic and estuarine environments. Mar Ecol Prog Ser 92:77–87
DeFlaun MF, Paul JH, Jeffrey WH (1987) Distribution and molecular weight of dissolved DNA in subtropical estuarine and oceanic environments. Mar Ecol Prog Ser 38:65–73
Faganelli J, Herndl G (1991) Dissolved organic matter in the waters of the Gulf of Trieste (Northern Adriatic). Thalassia Jugoslavica 23:51–63
Fenchel T (1982) Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar Ecol Prog Ser 9:35–42
Gasol MJ, Vaqué D (1993) Lack of coupling between heterotropic nanoflagellates and bacteria: a general phenomenon across aquatic systems? Limnol Oceanogr 38:657–665
González JM, Suttle CA (1993) Grazing by marine nanoflagellates on viruses and viral-sized particles: ingestion and digestion. Mar Ecol Prog Ser 94:1–10
Hara S, Terauchi K, Koike I (1991) Abundance of viruses in marine waters: assessment by epiflourescence and transmission electron microscopy. Appl Environ Microbiol 57:2731–2734
Heldal M, Bratbak G (1991) Production and decay of viruses in aquatic environments. Mar Ecol Prog Ser 72:205–212
Hobbie JE, Daley RJ, Jasper S (1977) Use of Nuclepore filters for counting bacteria by epifluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Ivancic I, Degobbis D (1987) Mechanisms of production and fate of organic phosphorous in the northern Adriatic Sea. Mar Biol 94:117–125
Jiang SC, Paul JH (1994) Seasonal and diel abundances of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar Ecol Prog Ser 104:163–172
Karl DM, Bailiff MD (1989) The measurement and distribution of dissolved nucleic acids in aquatic environments. Limnol Oceanogr 34:543–558
Maruyama A, Oda M, Higashihara T (1993) Abundance of virus-sized non-DNase-digestible (coated DNA) in eutrophic seawater. Appl Environ Microbiol 59:712–717
Mathews J, Buthala DA (1970) Centrifugal sedimentation of virus particles for electron microscopic counting. J Virol 5:598–603
Parsons T, Maita Y, Lalli C (1984) A Manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, UK
Paul JH, Carlson DJ (1984) Genetic material in the marine environment: implication for bacterial DNA. Limnol Oceanogr 29:1091–1097
Paul JH, DeFlaun MF, Jeffrey WH, David AW (1988) Seasonal and diel variability in dissolved DNA and in microbial biomass and activity in a subtropical estuary. Appl Environ Microbiol 54:718–727
Paul JH, Jeffrey WH, Cannon JP (1990) Production of dissolved DNA, RNA, and protein by microbiol populations in a Florida reservoir. Appl Environ Microbiol 56:2957–2962
Paul JH, Jiang SC, Rose JB (1991) Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol 57:2197–2204
Paul JH, Rose JB, Jiang SC, Kellogg CA, Dickson L (1993) Distribution of viral abundance in the reef environment of Key Largo, Florida. Appl Environ Microbiol 59:718–724
Peduzzi P, Weinbauer MG (1993) Effect of concentrating the virus-rich 2–200nm size fraction for the formulation of algal flocs (marine snow). Limnol Oceanogr 38:1562–1565
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62
Proctor LM, Fuhrman JA (1991) Roles of viral infection in organic particle flux. Mar Ecol Prog Ser 69:133–142
Proctor LM, Fuhrman JA (1992) Mortality of marine bacteria in response to enrichments of the virus size fraction from seawater. Mar Ecol Prog Ser 87:283–293
Reanney DC, Ackermann H-W (1982) Comparative biology and evolution of bacteriophages. Adv Viral Res 27:205–280
Sakano S, Kamatani A. (1992) Determination of dissolved nucleic acids in seawater by the fluorescence dye, ethidium bromide. Mar Chem 37:239–255
Sherr EB, Sherr BF (1987) High rates of consumption of bacteria by pelagic ciliates. Nature 325:710–711
Steward GF, Wikner J, Cochlan WP, Smith DC, Azam F (1992) Estimation of virus production in the sea. I. Method development. Mar Microb Food Webs 6:57–78
Steward GF, Wikner J, Cochlan WP, Smith DC, Azam F (1992) Estimation of virus production in the sea. II. Field results. Mar Microb Food Webs 6:79–90
Suttle CA, Chan AM, Cottrell MT (1990) Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347:467–469
Suttle CA, Chen F (1992) Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol 58:3721–3729
Turk V, Rehnstam A-S, Lundberg E, Hagström A (1992) Release of bacterial DNA by marine nanoflagellates, an intermediate step in phosphorus regeneration. Appl Environ Microbiol 58:3744–3750
Weinbauer MG, Fuks D, Peduzzi P (1993) Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl Environ Microbiol 59:4074–4082
Weinbauer MG, Peduzzi P (1994) Distribution, size and frequency of bacteriophages in different-bacterial morphotypes. Mar Ecol Prog Ser 108:11–20
Weinbauer MG, Peduzzi, P (in press) Comments on the determination of nucleic acids in natural waters by the CTAB-DABA-orcinol method. Sci. Total Environ.
Wikner J, Vallino JJ, Steward GF, Smith DC, Azam F (1993) Nucleic acids from the host bacterium as a major source of nucleotides for three marine bacteriophages. FEMS Microbiol Ecol 12:237–248
Wommack KE, Hill RT, Kessel M, Russek-Cohen E, Colwell RR (1992) Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol 58:2965–2970
Author information
Authors and Affiliations
Additional information
Correspondence to: M.G. Weinbauer
Rights and permissions
About this article
Cite this article
Weinbauer, M.G., Fuks, D., Puskaric, S. et al. Diel, seasonal, and depth-related variability of viruses and dissolved DNA in the northern Adriatic Sea. Microb Ecol 30, 25–41 (1995). https://doi.org/10.1007/BF00184511
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00184511