Abstract
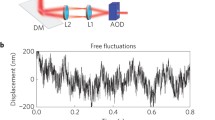
The undulatory excitations (flickering) of human and camel erythrocytes were evaluated by employing the previously used flicker spectroscopy and by local measurements of the autocorrelation function K (t) of the cell thickness fluctuations using a dynamic image processing technique. By fitting theoretical and experimental flicker spectra relative values of the bending elastic modulus K c of the membrane and of the cytoplasmic viscosity η were obtained. The effects of shape changes were monitored by simultaneous measurement of the average light intensity I 0 passing the cells and by phase contrast microscopic observation of the cells. Evaluation of the cellular excitations in terms of the quasi-spherical model yielded values of K c /R sup3inf0 and η · R 0 (R 0=equivalent sphere radius) and allowed us to account (1) for volume changes, (2) for effects of surface tension and spontaneous curvature and (3) for the non-exponential decay of K (t). From the long time decay of K (t) we obtained an upper limit of the bending elastic modulus of normal cells of K c = 2–3 · 10−19 Nm which is an order of magnitude larger than the value found by reflection interference contrast microscopy (RICT, K c , = 3.4 · 10−20 Nm, Zilker et al. 1987) but considerably lower than expected for a bilayer containing 50% cholesterol (K c = 5 · 10−19 Nm, Duwe et al. 1989). The major part of the paper deals with long time measurements (order of hours) of variations of the apparent K c and η values of single cells (and their reversibility) caused (1) by osmotic volume changes, (2) by discocytestomatocyte transitions induced by albumin and triflouperazine, (3) by discocyte-echinocyte transitions induced by expansion of the lipid/protein bilayer (by incubation with lipid vesicles) and by ATP-depletion in physiological NaCI solution, (4), by coupling or decoupling of bilayer and cytoskeleton using wheat germ agglutinin or erythrocytes with elliptocytosis and (5) by cross-linking the cytoskeleton using diamide. These experiments showed: (1) K c and η are minimal at physiological osmolarity and temperature and well controlled over a large range of these parameters. (2) Echinocyte formation does not markedly alter the apparent membrane bending stiffness. (3) During swelling the cell may undergo a transient discocyte-stomatocyte transition. (4) Strong increases of the apparent K c and η after cup-formation or strong swelling and deflation are due to the effect of shear elasticity and surface tension. Our major conclusions are: (1) The erythrocyte membrane exhibits a shear free deformation regime which requires ATP for its maintenance. (2) Shape transitions may be caused by relative area changes either of the two monolayers of the lipid/protein bilayer (corresponding to the bilayer coupling hypothesis) or of the bilayer and the cytoskeleton where the latter mechanism appears to be more frequent. (3) The low bending stiffness and the shear free deformation regime are explained in terms of a slight excess area of the lipid bilayer leading to a pre-undulated surface profile. Freeze fracture electron microscopy studies provide direct evidence for a pre-undulated bilayer with an undulation wavelength of approximately 100 nm.
Similar content being viewed by others
References
Backman L (1988) Functional or futile phosphorus? Nature 334:653–654
Bennett V (1989) The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta 988:102–121
Bessis M, Mohandas N (1975) A diffractometric method for the measurement of cellular deformability. Blood Cells 1:307–313
Brochard F, Lennon JF (1975) Frequency spectrum of the flicker phenomenon in erythrocytes. J Phys 36:1035–1047
Bull BS, Weinstein RS, Korpman RA (1986) On the thickness of the red cell membrane skeleton: quantitative electron microscopy of maximally narrowed isthmus regions of intact cells. Blood Cells 12:25–42
Byers TJ, Branton D (1985) Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci USA 82:6153–6157
Canham PB (1970) The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J Theor Biol 26:61–81
Chasis JA, Mohandas N, Shohet SB (1985) Erythrocyte membrane rigidity induced by Glycophorin A ligand interaction — evidence for an ligand-induced association between Glycophorin A and skeletal proteins. J Clin Invest 75:1919–1926
Cohen CM, Foley SF (1980) Phorbol ester- and Ca++-dependent phosphorylation of human red cell membrane skeletal proteins. J Biol Chem 261:7701–7709
Dall'Asta V, Gazzola GC, Longo N et al. (1986) Perturbation of Na+- and K+-gradients in human fibroblasts incubated in unsupplemented saline solutions. Biochim Biophys Acta 860:1–8
Deeley JOT, Coakley WT (1983) Interfacial instability and membrane internalization in human erythrocytes heated in the presence of serum albumin. Biochim Biophys Acta 727:293–302
De Gennes PG (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca
Deuling HJ, Helfrich W (1976) The curvature elasticity of fluid membranes: a catalogue of vesicles shapes. J Phys 37:1335–1345
Duwe H-P, Engelhardt H, Zilker A, Sackmann E (1987) Curvature elasticity of smectic A lipid bilayers and cell plasma membranes. Mol Cryst Liq Cryst 152:1–7
Duwe H-P, Eggle P, Sackmann E (1989) The cell-plasma membranes as a composite system of two-dimensional liquid crystal and macromolecular network and how to mimic its physical properties. Angew Makromol Chem 166:1–19
Engelhardt H, Sackmann E (1988) On the measurement of shear elastic moduli and viscosities of erythrocyte plasma membranes by transient deformation in high frequency electric fields. Biophys J 54:495–508
Evans EA (1973) Bending resistance and chemically induced bending moments in membrane bilayers. Biophys J 14:923–931
Evans E, Needham D (1986) Giant vesicle bilayers composed of mixtures of lipids, cholesterol and polypeptids. Faraday Discuss Chem Soc 81:267–280
Evans EA, Parsegian VA (1983) Energetics of membrane deformation and adhesion in cell and vesicle aggregation. Ann NY Acad Sci 416:13–26
Evans E, Skalak R (1980) Mechanics and thermodynamics of biomembranes. CRC Press, Boca Raton, Fla
Fricke K, Sackmann E (1984) Variation of frequency spectrum of the erythrocyte flickering caused by aging, osmolarity, temperature and pathological changes. Biochim Biophys Acta 803:145–152
Fricke K, Wirthensohn K, Laxhuber R, Sackmann E (1986) Flicker spectroscopy of erythrocytes. Eur Biophys J 14:67–81
Golan DE, Veach W (1980) Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery. Evidence for control by cytoskeletal interactions. Proc Natl Acad Sci USA 77:2537–2541
Helfrich W (1973) Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch 28c:693–703
Helfrich W (1978) Steric interaction of fluid membranes in multilayer systems. Z Naturforsch 33a:305–315
Hochmut RM, Worthy PR, Evans EA (1979) Red cell extensional recovery and the determination of membrane viscosity. Biophys J 26:101–114
Kapitza H-G, Sackmann E (1980) Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta 595:56–64
Khodadad JK, Weinstein RS (1983) The band 3-rich membrane of llama erythrocytes: studies on cell shape and the organization of membrane proteins. J Membr Biol 72:161–171
Leibler S, Singh RRP, Fisher ME (1987) Thermodynamic behavior of two-dimensional vesicles. Phys Rev Lett 59:1989–1992
Liu SC, Derick LH, Palek J (1987) Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol 104:527–536
Marquardt DW (1963) An algorithm for least square estimation of nonlinear parameters. J Soc Industr Appl Math 11:431–441
Milner ST, Safran SA (1987) Dynamical fluctuations of droplet microemulsions and vesicles. Phys Rev A 36:4371–4379
Nelson DR, Halperin BI (1979) Dislocation-mediated melting in two dimensions. Phys Rev B 19:2457–2483
Nelson GA, Andrews LA, Karnovsky MJ (1983) Control of erythrocyte shape by calmodulin. J Cell Biol 96:730–735
Ott P, Hope MJ, Verkleij AJ, Roelofsen B, Brodbeck U, van Deenen LLM (1981) Effect of dimyristoyl phosphatidylcholine on intact erythrocytes. Biochim Biophys Acta 641:79–87
Petersen NO, McConnaughey WB, Elson EL (1982) Dependence of locally measured cellular deformability on position on the cell, temperature and Cytochalasin B. Proc Natl Acad Sci USA 79:5327–5331
Peterson MA (1985) Shape dynamics of nearly spherical membrane bounded fluid cells. Mol Cryst Liq Cryst 127:257–263
Petrov AG, Seleznev SA, Derzhanski A (1979) Principles and methods of liquid crystal physics applied to the structure and function of biological membranes. Acta Phys Pol A 55:385–405
Sackmann E, Duwe HP (1990) Bending elasticity and thermal excitations of lipid bilayer vesicles. Physica (in press)
Schneider MB, Jenkins JT, Webb WW (1984) Thermal fluctuations of large quasi-spherical bimolecular phospholipid vesicles. J Phys 45:1457–1472
Sheetz PM, Singer SJ (1974) Biological membranes as bilayer couples. A molecular mechanism of drug — erythrocyte interactions. Proc Natl Acad Sci USA 71:4457–4461
Shen BW, Josephs R, Steck TL (1984) Ultrastructure of unit fragments of the skeleton of the human erythrocyte membrane. J Cell Biol 99:810–821
Shohet SB, Nathan DG (1970) Incorporation of phosphatide precursors from serum into erythrocytes. Biochim Biophys Acta 202:202–205
Smith JE, Mohandas N, Shohet SB (1979) Variability in erythrocyte deformability among various mammals. Am J Physiol 236:H725-H730
Steck TL (1989) Red cell shape. In: Stein W, Bronner F (eds) cell shape: determinants, regulation and regulatory role. Academic Press, New York
Stokke BT, Mikkelsen A, Elgsaeter A (1986) Spectrin, human erythrocyte shapes and mechanochemical properties. Biophys J 49:319–327
Svetina S, Zeks B (1989) Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J 17:101–111
Takakuwa Y, Tchernia G, Rossi M, Benabadji M, Mohandas N (1986) Restoration of normal membrane stability to unstable protein 4.1 deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest 78:80–85
Waugh RE (1987) Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J 51:363–369
Yoshino H, Minari O (1987) Heat-induced dissociation of human erythrocyte spectrin dimer into monomers. Biochim Biophys Acta 905:100–108
Zilker A, Engelhardt H, Sackmann E (1987) Dynamic reflection interference contrast (RIC-) microscopy: a new method to study surface excitations of cells and to measure membrane bending elastic moduli. J Phys 48:2139–2151
Author information
Authors and Affiliations
Additional information
Offprint requests to: E. Sackmann
Rights and permissions
About this article
Cite this article
Zeman, K., Engelhard, H. & Sackmann, E. Bending undulations and elasticity of the erythrocyte membrane: effects of cell shape and membrane organization. Eur Biophys J 18, 203–219 (1990). https://doi.org/10.1007/BF00183373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00183373