Abstract
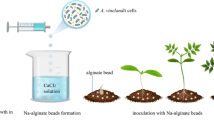
Alginate polymer was evaluated as a carrier for seed inoculation with a genetically modified strainPseudomonas fluorescens F113LacZY, which protects sugar-beet againstPythium-mediated damping-off. F113LacZY survived in alginate beads at 5 log10 CFU/ bead or higher counts for 8 weeks of storage, regardless of the conditions of incubation. In plant inoculation experiments, colonisation of the growing area of the root by F113LacZY, derived from alginate beads placed in the soil next to the seed or from an alginate coating around the seeds, was improved compared with application of just free cells of the strain. F113LacZY trapped in alginate beads was an effective producer of antifungal phloroglucinols as indicated by direct HPLC quantification of phloroglucinols and in vitro inhibition of both the indicator bacteriumBacillus subtilis A1 and the pathogenic fungusPythium ultimum. Alginate polymer represents a promising carrier for the delivery of biocontrol inoculants for root colonisation and production of antifungal metabolites.
Similar content being viewed by others
References
Audet P, Paquin C, Lacroix C (1989) Sugar utilization and acid production by free and entrapped cells ofStreptococcus salivarius subsp.thermophilus, Lactobacillus delbrueckii subsp.bulgaricus andLactococcus lactis subsp.lactis in a whey permeate medium. Appl Environ Microbiol 55:185–189
Bandyopadhyay A, Das AK, Mandal SK (1993) Erythromycin production byStretomyces erythureus entrapped in calcium alginate beads. Biotechnol Lett 15:1003–1006
Cheetham PSJ, Blunt KW, Bucke C (1979) Physical studies on cell immobilization using calcium alginate gels. Biotechnol Bioeng 21:2155–2168
DeLucca II AJ, Connick Jr WJ, Fravel DR, Lewis JA, Bland JM (1990) The use of bacterial alginates to prepare biocontrol formulations. J Ind Microbiol 6:129–134
Dowling DN, O'Gara F (1994) Metabolites ofPseudomonas involved in the biocontrol of plant disease. Trends Biotechnol 12:133–141
Elsas JD van, Trevors JT, Jain DK, Wolters AC, Heijnen CE, Overbeek LS van (1992) Survival of, and root colonization by, alginate-encapsulatedPseudomonas fluorescens cells following introduction into soil. Biol Fertil Soils 14:14–22
Fenton AM, Stephens PM, Crowley J, O'Callaghan M, O'Gara F (1992) Exploitation of gene(s) involved in 2,4-diacetylploroglucinol biosynthesis to confer a new biocontrol capability to aPseudomonas strain. Appl Environ Microbiol 58:3873–3878
Fravel DR, Marois JJ, Lumsden RD, Connick Jr WJ (1985) Encapsulation of potential biocontrol agents in an alginate-clay matrix. Phytopathology 75:774–777
Frioni L, Le Roux C, Dommergues YR, Diem HG (1994) Inoculant made of encapsulatedFrankia: assessment ofFrankia growth within alginate beads. World J Microbiol Biotechnol 10:118–121
Herrero M, De Lorenzo V, Timmis KN (1990) Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gramnegative bacteria. J Bacteriol 172:6557–6567
Jung G, Mugnier J, Diem HG, Dommergues YR (1982) Polymer entrappedRhizobium as an inoculant for legumes. Plant Soil 65:219–231
Keel C, Voisard C, Berling CH, Kadr G, Défago G (1989) Iron sufficiency, a prerequisite for the suppression of tobacco root rot byPseudomonas fluorescent strain CHA0 under gnotobiotic conditions. Phytopathology 79:584–589
Kierstan M, Bucke C (1977) The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels. Biotechnol Bioeng 19:387–397
Kloepper JW, Beauchamp CJ (1992) A review of issues related to measuring colonization of plant roots by bacteria. Can J Microbiol 28:1219–1232
Loper JE, Suslow TV, Schroth MN, (1984) Lognormal distribution of bacterial populations in the rhizosphere. Phytopathology 74:1454–1460
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Martinsen A, Skjak-Braek G, Smidsrod O (1989) Alginate as immobilization material. I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol Bioeng 33:78–89
Mugnier J, Jung G (1985) Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer gels. Appl Environ Microbiol 50:108–114
O'Sullivan DJ, O'Gara F (1992) Traits of fluorescentPseudomonas spp. involved in suppression of plant root pathogens. Microbial Rev 56:662–676
Paul E, Fages J, Blanc P, Goma G, Pareilleux A (1993) Survival of alginate-entrapped cells ofAzospirillum lipoferum during dehydration and storage in relation to water properties. Appl Microbiol Biotechnol 40:34–39
SAS Institute Inc (1985) SAS user's guide: statistics. SAS Institute, Cary, NC
Scher FM, Baker R (1982) Effect ofPseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness toFusarium wilt pathogens. Phytopathology 72:1567–1573
Shanahan P, O'Sullivan DJ, Simpson P, Glennon JD, O'Gara F (1992) Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol 58:353–358
Shanahan P, Glennon JD, Crowley J, Donnelly DF, O'Gara F (1993) Liquid chromatograpic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta 272:271–277
Thomashow LS, Weller D (1988) Role of phenazine antibiotic fromPseudomonas fluorescens in biological control ofGaeumannomyces graminis var.tritici. J Bacteriol 170:3499–3508
Trevors JT, Elsas JD van, Lee H, Overbeek LS van (1992) Use of alginate and other carriers for encapsulation of microbial cells for use in soil. Microbial Rel 1:61–69
Trevors JT, Elsas JD van, Lee H, Wolters AC (1993) Survival of alginate-encapsulatedPseudomonas fluorescens cells in soil. Appl Microbiol Biotechnol 39:637–643
Vilchez C, Vega JM (1994) Nitrite uptake byChlamydomonas reinhardtii cells immobilized in calcium alginate. Appl Microbiol Biotechnol 41:137–141
Zezza N, Pasini G, Lombardi A, Mercenier A, Spettoli P, Zamorani A, Nuti MP (1993) Production of a bacteriocin active on lactate-fermenting clostridia byLactococcus lactis subsp.lactis immobilized in coated alginate beads. J Diary Res 60:581–591
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Russo, A., Moënne-Loccoz, Y., Fedi, S. et al. Improved delivery of biocontrolPseudomonas and their antifungal metabolites using alginate polymers. Appl Microbiol Biotechnol 44, 740–745 (1996). https://doi.org/10.1007/BF00178612
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00178612