Summary
Cell motility within central nervous system (CNS) neuropil may be largely restricted yet infiltration by glioma cells is commonly observed. Glioma cells remodel nervous tissue and may assemble extracellular matrix in order to migrate. We examined the rat C6 glioma cell line for laminin expression and response in vitro and following engraftment into rat spinal cord. C6 cell cultures expressed laminin-2. C6 cells attached equally well to substrates of purified laminin-1 and laminin-2 and laminin-2-enriched C6 conditioned medium. In contrast, C6 cell migration was substantially greater on laminin-2 and C6-derived substrata than on laminin-1. Glioma cell attachment to laminin-1 and -2 was largely inhibited by antibody to the laminin receptor LBP110 and by an IKVAV peptide but not by YIGSR or control peptides. IKVAV peptide and anti-LBP110 antibodies also inhibited glioma cell invasion through synthetic basement membrane. Anti-β1 integrin antibody selectively inhibited cell migration and invasion on laminin-2 substrata without affecting percent cell attachment. These findings suggest C6 cell migration and invasion are promoted by autocrine release of laminin-2 and involve LPB110 and β1 integrin laminin receptors.
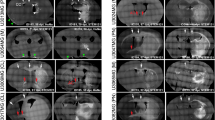
A possible role for laminin-2 in CNS infiltration in vivo was examined following glioma engraftment into rat spinal cord. Engrafted C6 tumors share many histologic features of invasive human glioma. Engrafted glioma cells expressed laminin, LBP110 and β1 integrin antigens, indicating the molecular mechanisms of C6 motility observed in culture may contribute to glioma invasion in vivo. NMR and corroborative immunocytochemistry provided precise means to monitor tumor progression following glioma engraftment into rat spinal cord. Advantages of this glioma model are discussed regarding the assessment of anti-adhesive therapies in vivo.
Similar content being viewed by others
References
Benda P, Lightbody J, Sato G, Leving J, Sweet W: Differentiated rat glial cell strain in culture. Science 161: 370–371, 1968
Bernier SM, Utani A, Sugiyama S, Doi T, Polistina C, Yamada Y: Cloning and expression of Laminin alpha2 chain (M-chain) in the mouse. Matrix Biol 14: 447–455, 1995
Bernstein JJ, Goldberg WJ, Laws ER: Human malignant astrocytoma xenografts migrate in rat brain: a model for central nervous system cancer research. J Neurosci Res 22:134–143, 1989
Bernstein JJ, Goldberg WJ, Laws EJ, Conger D, Morreale V, Wood LR: C6 glioma cell invasion and migration of rat brain after neural homografting: ultrastructure. Neurosurgery 26: 622–628, 1990
Bernstein JJ, Laws EJ, Levine KV, Wood LR, Tadvalkar G, Goldberg WJ: C6 glioma-astrocytoma cell and fetal astrocyte migration into artificial basement membrane: a permissive substrate for neural tumors but not fetal astrocytes. Neurosurgery 28: 652–658, 1991
Buck C, Albelda S, Damjanovich L, Edelman J, Shih DT, Solowska J: Cell-matrix contacts and pericellular proteolysis. Immunohistochemical and molecular analysis of betal and beta3 integrins. Cell Differ Dev 32: 189–202, 1990
Charonis AS, Skubitz AP, Koliakos GG, Reger LA, Dege J, Vogel AM, Wohlhueter R, Furcht LT: A novel synthetic peptide from the B1 chain of laminin with heparin-binding and cell adhesion-promoting activities. J Cell Biol 107: 1253–1260, 1988
Chin AY, de los Monteros E, Cole RA, Loera S, de Vellis J: Laminin and s-laminin are produced and released by astrocytes, Schwann cells, and schwannomas in culture. Glia 4: 11–24, 1991
Dausse E, Quemeneur E, Schwartz K: Heat-treated bovine serum albumin is a better blocking reagent for adhesion studies. Biotechniques 18: 430–431, 1995
Ehrig K, Leivo J, Argraves WS, Ruoslahti E, Engvall E: Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA 87: 3264–3268, 1990
Engvall E, Krusius T, Wewer V, Ruoslahti E: Laminin from rat yolk sac tumor: isolation, partial characterization and comparison with mouse laminin. Arch Biochem Biophys 222: 649–656, 1983
Engvall E, Earwicker D, Day A, Muir D, Manthorpe M, Paulsson M: Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 schwannoma cells. Exp Cell Res 198: 115–123, 1992
Gehlsen KR, Argraves WS, Pierschbacher MD, Ruoslahti E: Inhibition of in vitro tumor cell invasion by Arg-Gly-Aspcontaining synthetic peptides [published erratum appears in J Cell Biol 1989 Jun; 108 (6): following 2546]. J Cell Biol 106: 925–930, 1988
Giese A, Rief MD, Loo MA, Berens ME: Determinants of human astrocytoma migration. Cancer Res 54: 3897–3904, 1994
Giese A, Rief MD, Tran NL, Berens ME: Specific attachment and migration of human astrocytoma cells on human but not murine laminin. Glia 13: 64–74, 1995
Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, Robey FA, Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell 48: 989–996, 1987
Jucker M, Walker LC, Kibbey MC, Kleinman HK, Ingram DK: Localization of a laminin-binding protein in brain. Neuroscience 56: 1009–1022, 1993
Kaye A, Morstyn G, Gardner I, Pyke K: Development of a xenograft glioma model in mouse brain. Cancer Res 46: 1367–1373, 1986
Kibbey MC, Jucker M, Weeks BS, Neve RL, Van NW, Kleinman HK: beta-amyloid precursor protein binds to the neurite-promoting IKVAV site of laminin. Proc Natl Acad Sci USA 90: 10150–10153, 1993
Kim WH, Schnaper HW Nomizu M, Yamada Y, Kleinman HK: Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res 54: 5005–5010, 1994
Kleinman HK, McGarvey ML, Hassel JR, Star VL, Cannon FB, Laurie GW, Martin GR: Basement membrane complexes with biological activity. Biochem 25: 312–318, 1986
Kleinman HK, Weeks BS, Cannon FB, Sweeney TM, Sephel GC, Clement B, Zain M, Olson M, Jucker M, Burrows BA: Identification of a 110-kDa nonintegrin cell surface laminin-binding protein which recognizes an A chain neurite-promoting peptide. Arch Biochem Biophys 290: 320–325, 1991
Koochekpour S, Merzak A, Pilkington GJ: Extracellular matrix proteins inhibit proliferation, upregulate migration and induce morphological changes in human glioma cell lines. Eur J Cancer [A] 31A: 375–380, 1995
LeBeau JM, Liuzzi FJ, Depto AS, Vinik AI: Differential laminin gene expression in dorsal root ganglion neurons and nonneuronal cells. Exp Neurol 127: 1–8, 1994
Leivo I, Engvall E: Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Nall Acad Sci USA 85: 1544–1548, 1988
Leivo J, Engvall E, Laurila P, Miettinen M: Distribution of merosin, a laminin-related tissue-specific basement membrane protein, in human Schwann cell neoplasms. Lab Invest 61: 426–432, 1989
Liesi P: Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J 4: 2505–2511, 1985
Luckenbill-Edds L, Kaiser CA, Rodgers TR, Powell DD: Localization of the 110 kDa receptor for laminin in brains of embryonic and postnatal mice. Cell Tissue Res 279:371–377, 1995
Maher JJ, Tzagarakis C: Partial cloning of the M subunit of laminin from adult rat lipocytes: expression of the M subunit by cells isolated from normal and injured liver. Hepatology 19: 764–770, 1994
Malek-Hedayat S, Rome LH: Expression of multiple integrins and extracellular matrix components by C6 glioma cells. J Neurosci Res 31: 470–478, 1992
Maria BL, Eskin TA, Quisling RG: Brainstem glioma and other malignant gliomas: II. Possible mechanisms of brain infiltration by tumor cells. J Child Neurol 8: 292–305, 1993
McComb RD, Bigner DD: Immunolocalization of laminin in neoplasms of the central and peripheral nervous systems. J Neuropathol Exp Neurol 44: 242–253, 1985
McKeever PE, Fligiel SE, Varani J, Castle RL, Hood TW: Products of cells cultured from gliomas. VII. Extracellular matrix proteins of gliomas which contain glial fibrillary acidic protein. Lab Invest 60: 286–295, 1989
Muir D, Sukhu L, Johnson J, Lahorra MA, Maria BL: Quantitative methods for scoring cell migration and invasion in filter-based assays. Anal Biochem 215: 104–109, 1993
Muir D: Metalloproteinase-dependent neurite outgrowth within a synthetic extracellular matrix is induced by nerve growth factor. Exp Cell Res 210: 243–252, 1994
Nagano N, Sasaki H, Aoyagi M, Hirakawa K: Invasion of experimental rat brain tumor: Early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol Berl 86: 117–125, 1993
Paulsson M, Saladin K: Mouse heart laminin. Purification of the native protein and structural comparison with Engelbreth-Holm-Swarm tumor laminin. J Biol Chem 264: 18726–18732 Issn: 0021–9258, 1989
Paulus W, Tonn JC: Interactions of glioma cells and extracellular matrix. J Neuro-Oncol 24: 87–91, 1995
Pedersen PH, Marienhagen K, Mork S, Bjerkvig R: Migratory pattern of fetal rat brain cells and human glioma cells in the adult rat brain. Cancer Res 53: 5158–5165, 1993
Rutka JT, Myatt CA, Giblin JR, Davis RL, Rosenblum ML: Distribution of extracellular matrix proteins in primary human brain tumours: an immunohistochemical analysis. Can J Neurol Sci 14: 25–30, 1987
Rutka JT, Apodaca G, Stern R, Rosenblum M: The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg 69: 155–170, 1988
Shea TB: Secretion of neurite-promoting factors by astroglial cells is a function of glial differentiation state rather than age. Neurosci Res Commun 15: 119–123, 1994
Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, Yamada Y: A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem 264: 16174–16182, 1989
Tashiro K, Sephel GC, Greatorex D, Sasaki M, Shirashi N, Martin GR, Kleinman HK, Yamada Y: The RGD containing site of the mouse laminin A chain is active for cell attachment, spreading, migration and neurite outgrowth. J Cell Physiol 146: 451–459, 1991
Terranova VP, Williams JE, Liotta LA, Martin GR: Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science 226: 982–985, 1984
Timpl R, Rohde H, Robey PG, Rennard SI, Foidart J, Martin GR: Laminin-A glycoprotein from basement membranes. J Biol Chem 254: 9933–9937, 1979
Timpl R, Brown JC: The laminins. Matrix 14: 275–281, 1994
Turpeenniemi-Hujanen T, Thorgeirsson UP, Rao CN, Liotta LA: Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem 261: 1883–1889, 1986
Westermann R, Mollenhauer J, Johannsen M, Unsicker K: Laminin and other basal lamina proteins with neurite promoting activity in medium conditioned by C6 glioma cells. Int J Dev Neurosci 7: 219–230, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muir, D., Johnson, J., Rojiani, M. et al. Assessment of laminin-mediated glioma invasion in vitro and by glioma tumors engrafted within rat spinal cord. J Neuro-Oncol 30, 199–211 (1996). https://doi.org/10.1007/BF00177271
Issue Date:
DOI: https://doi.org/10.1007/BF00177271