Abstract
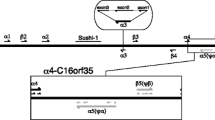
Artemia has evolved the longest known concatenation of hemoglobin domains, the α subunit containing nine domains and the β subunit having a similar size. Translation of the cDNA sequence of the α subunit reveals eight regions of inter-domain polypeptide linking together the nine heme-binding domains, together with partially analogous sequences preceding the first domain and following the last. Analysis of the structural possibilities of the linker sequences suggests how the domains may be organized in the subunit.
The interdomain linker sequences were 14%–64% identical (62%–91% similar by Dayhoff substitution matrix) and approximately 14 residues in length including a consensus -Val-Asp-Pro-Val-Thr-Gly-Leu-. The linker composition resembled that of the 11 amino acid pre-A leader sequence of Petromyzon marinus (lamprey) hemoglobin V, the structure of which is known. Prediction of structure from the Artemia linker sequences indicated a nonhelical, turn-associated linker which could be modeled to the Petromyzon leader. Measurements confirmed that such a structure could support the packing of nine Artemia domains into a polymeric subunit of annular shape, two of which subunits (which can be similar or dissimilar) comprise the physiological molecule.
The position of interdomain introns and the character of a variable residue early in the linker are compatible with the nine-domain polymer having evolved through gene duplication reflected in globin domain fusion incorporating an extension specifically of the N-terminus. The multiplication of an original single-domain globin gene to give the present nine is estimated from sequence differences, allowing for multiple mutations at individual sites, to have occurred in a period at least 500–700 million years ago.
Similar content being viewed by others
References
Argos P (1990) An investigation of oligopeptides linking domains in protein tertiary structures and possible candidates for general gene fusion. J Mol Biol 211:943–958
Bashford D, Chothia C, Lesk AM (1987) Determinants of a protein fold. Unique features of the globin amino acid sequences. J Mol Biol 196:199–216
Bogusz D, Appleby CA, Landsmann J, Dennis ES, Trinick MJ, Peacock WJ (1988) Functioning haemoglobin genes in non-nodulating plants. Nature 331:178–180
Como PF, Thompson EOP (1980) Amino acid sequence of the α chain of the tetrameric haemoglobin of the bivalve mollusc Anadara trapezia. Aust J Biol Sci 38:221–236
Dayhoff MO, Barker WC, Hunt LT (1983) Establishing homologies in protein sequences. In: Hirs CHW, Timasheff SN (eds) Methods in enzymology, vol 91. Academic Press, New York, pp 524–545 1444 0939 V 3
De Baere I, Liu L, Moens L, Van Beeumen J, Gielens C, Richelle J, Trotman CNA, Finch J, Gerstein M, Perutz M (1992) Polar zipper sequence in the high-affinity hemoglobin of Ascaris suum: amino acid sequence and structural interpretation. Proc Nat Acad Sci USA 89:4638–4642
Dickerson RE (1971) The structure of cytochrome c and the rates of molecular evolution. J Mol Evol 1:26–45
Dixon B, Walker B, Kimmins W, Pohajdak B (1991) Isolation and sequencing of a cDNA for an unusual hemoglobin from the parasitic nematode Pseudoterranova decipiens. Proc Nat Acad Sci USA 88:5655–5659
Felsenstein J (1988) Phylogenies from molecular sequences: inference and reliability. Ann Rev Genet 22:521–565
Fisher WK, Gilbert AT, Thomson EOP (1984) Amino acid sequence of the globin IIB chain of the dimeric haemoglobin of the bivalve mollusc Anadara trapezia. Aust J Biol Sci 37:191–203
Furuta H, Kajita A (1983) Dimeric hemoglobin of the bivalve mollusc Anadara broughtonii: Complete amino acid sequence of the globin chain. Biochemistry 22:917–922
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120:97–120
Gilbert AT, Thompson EOP (1985) Amino acid sequence of the β-chain of the tetrameric haemoglobin of the bivalve mollusc, Anadara trapezia. Aus J Biol Sci 38:221–236
Goodman M, Pedwaydon J, Czelusniak J, Suzuki T, Gotoh T, Moens L, Shishikura F, Walz D, Vinogradov S (1988) An evolutionary tree for invertebrate globin sequences. J Mol Evol 27:236–249
Hombrados I, Rodewald K, Neuzil E, Braunitzer G (1983) Haemoglobins, LX. Primary structure of the major haemoglobin of the sea lamprey Petromyzon marinus (var. Garonne, Loire). Biochimie 65:247–257
Honzatco RB, Hendrickson WA, Love WE (1985) Refinement of a molecular model for Lamprey hemoglobin from Petromyzon marinus. J Mol Biol 184:147–164
Jellie AM, Bray JA, Tate WP, Trotman CNA (1991)Gene structure of an Artemia haemoglobin domain. Proc Univ Otago Med Sch 69:45–46
Jhiang SM, Riggs AF (1989) The structure of the gene encoding chain c of the hemoglobin of the earthworm, Lumbricus terrestris. J Biol Chem 264:19003–19008
Kortt AA, Trinick MJ, Appleby CA (1988) Amino acid sequences of hemoglobins I and 11 from root nodules of the non-leguminous Parasponia rigida-rhizobium symbiosis, and a correction of the sequence of hemoglobin I from Parasponia andersonii. Eur J Biochem 175:141–149
Manning AM, Marshall CJ, Powell RJ, Trotman CNA, Tate WP (1989) The molecular biology of Artemia haemoglobins. In: Warner AH, Bagshaw JC, MacRae TH (eds) Cell and molecular biology of Artemia development, NATO Advanced Study Institute Series A: Life Sciences. Plenum Press, New York, pp 413–425
Manning AM, Powell RJ, Trotman CNA, Tate WP (1990a) Developmental expression and cDNA cloning of globin genes from the brine shrimp, Artemia. New Biol 2:77–83
Manning AM, Trotman CNA, Tate WP (1990) Evolution of a polymeric globin in the brine shrimp Artemia. Nature 348:653–656
Mauri F, Omnaas J, Davidson L, Whitfill C, Kitto GB (1991) Amino acid sequence of a globin from the sea cucumber Caudina (Molpadia) arenicola. Biochim Biophys Acta 1078:63–67
Moens L, Vanfleteren J, De Baere I, Jellie AM, Tate WP, Trotman CNA (1992) Unexpected intron location in non-vertebrate globin genes. FEBS Lett 312:105–109
Moens L, Van Hauwaert M-L, Geelen D, Verpooten G, Van Beeuman J (1987) The structure of Artemia sp. haemoglobins I. The amino acid sequence of a structural unit. In: Decleir W, Moens L, Slegers H, Jaspers E, Sorgeloos P (eds) Artemia research and its applications, vol 2. Universa Press, Wetteren, Belgium, pp 93–98
Moens L, Van Hauwaert M-L, De Smet K, Geelen D, Verpooten G, Van Beeumen J, Wodak S, Alard P, Trotman CNA (1988) A structural domain of the covalent polymer globin chains of Artemia. Interpretation of amino acid sequence data. J Biol Chem 263:4679–4685
Moens L, Van Hauwaert M-L, De Smet K, Ver Donck K, Van De Peer Y, Van Beeumen J, Wodak S, Alard P, Trotman CNA (1990) Structural interpretation of the amino acid sequence of a second domain from the Artemia covalent polymer globin. J Biol Chem 265:14285–14291
Moens L, Wolf G, Van Hauwaert M-L, De Baere I, Van Beeumen J, Wodak S, Trotman CNA (1991) The extracellular hemoglobins of Artemia. Structure of the oxygen carrier and respiration physiology. In: Browne RA, Sorgeloos P, Trotman CNA (eds) Artemia biology. CRC Press, Boca Raton, pp 187–219
Naito Y, Riggs CL, Vandergon TL, Riggs AF (1991) Origin of a “bridge” intron in the gene for a two-domain globin. Proc Nat Acad Sci USA 88:6672–6676
Petruzelli R, Boffi A, Barra D, Bossa F, Ascoli F, Chiancone E (1989) Scapharca hemoglobins, type cases of a novel mode of chain assembly and heme-heme interaction. Amino acid sequence and subunit interaction of the tetrameric component. FEBS Lett 259:133–136
Petruzelli R, Gofffredo BM, Barra D, Bossa F, Boffi A, Verzilli D, Ascoli F, Chiancone E (1985) Amino acid sequence of the cooperative homodimeric hemoglobin from the mollusc Scapharca inaequivalvis and topology of the intersubunit contacts. FEBS Lett 184:328–332
Potts M, Angeloni SV, Ebel RE, Rassam D (1992) Myoglobin in a cyanobacterium. Science 256:1690–1692
Rawlings N, Ashman K, Wittmann-Liebold B (1983) Computerised version of the Chou and Fasman protein secondary structure predictive method. Int J Pept Protein Res 22:515–524
Richmond TJ, Richards FM (1978) Packing of α-helices: geometrical constraints and contact areas. J Mol Biol 119:537–555
Riggs C, Riggs AF (1990) cDNA-derived amino acid sequences of single and two-domain globins from the clam Barbatia reeveana. In: Preaux G, Lontie R (eds) Invertebrate dioxygen carriers. Leuven University Press, Leuven, pp 57–60
Royer WE, Hendrickson WA, Chiancone E (1989) The 2.4-Å crystal structure of Scapharca dimeric hemoglobin. Cooperativity based on directly communicating hemes at a novel subunit interface. J Biol Chem 264:21052–21061
Stockwell PA (1988) HOMED: a homologous sequence editor. Trends Biochem Sci 13:322–324
Suzuki T (1989) Amino acid sequence of the major globin from the sea cucumber Paracaudina chilensis. Biochim Biophys Acta 998:292–296
Trotman CNA, Manning AM, Moens L, Tate WP (1991) The polymeric haemoglobin molecule of Artemia. Interpretation of translated cDNA sequence of nine domains. J Biol Chem 266:13789–13795
Wakabayashi S, Matsubara H, Webster DA (1986) Primary sequence of a dimeric bacterial hemoglobin from Vitreoscilla. Nature 322:481–483
Wood EJ, Barker C, Moens L, Jacob W, Heip J, Kondo M (1981) Biophysical characterization of Artemia salina (L.) extracellular haemoglobins. Biochem J 193:353–359
Author information
Authors and Affiliations
Additional information
Correspondence to: C.N.A. Trotman 1444
Rights and permissions
About this article
Cite this article
Trotman, C.N., Manning, A.M., Bray, J.A. et al. Interdomain linkage in the polymeric hemoglobin molecule of Artemia . J Mol Evol 38, 628–636 (1994). https://doi.org/10.1007/BF00175883
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00175883