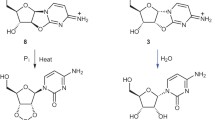
Abstract
Urazole is α five-membered heterocyclic compound which is isosteric with uracil's hydrogen-bonding segment. Urazole reacts spontaneously with ribose (and other aldoses) to give α mixture of four ribosides: α and β pyranosides and furanosides. This reaction occurs in aqueous solution at mild temperatures. Thermodynamic and kinetic parameters for the reaction of urazole with ribose were determined. In contrast, uracil is completely unreactive with ribose under these conditions. Urazole's unusual reactivity is ascribed to the hydrazine portion of the molecule. Urazole can be synthesized from biuret and hydrazine under prebiotic conditions. The prebiotic synthesis of guanazole, which is isosteric in part to diaminopyrimidine and cytosine, is accomplished from dicyandiamide and hydrazine. Kinetic parameters for both prebiotic reactions were measured. Urazole and guanazole are transparent in the UV, which would be α favorable property in the absence of an ozone layer on the early Earth. Urazole makes hydrogen bonds with adenine in DMSO similar to those of uracil, as established by IH NMR. All of these properties make urazole an attractive potential precursor to uracil and guanazole a potential precursor to cytosine in the RNA or pre-RNA world.
Similar content being viewed by others
References
Bausch MJ, David B, Dobrowolski P, Guadalupe-Fasano C, Gostowski R, Selmarten D, Prasad V, Vaughn A, Wang L-H (1991) Proton-transfer chemistry of urazoles and related imides, amides, and diacyl hydrazides. J Org Chem 56:5643–5651
Bose R, Yamada EW (1974) Uridine phosphorylase, molecular properties and mechanism of catalysis. Biochemistry 13:2051–2056
Breitmaier E, Hollstein U (1976) Complete assignment of the 13C n.m.r. spectrum of mutarotated D-ribose by integration and specific deuteration. Org Magn Reson 8:573–575
Camici M, Sgarrella F, Ipata PL, Mora U (1980) The standard Gibbs free energy change of hydrolysis of a α-d-ribose 1-phosphate. Arch Biochem Biophys 205:191–197
Chang C-J, Diaz LE, Woolfenden WR, Grant DM (1982) Solid-state carbon-13 nuclear magnetic resonance study of ribonucleosides and ribonucleic acid. J Org Chem 47:5318–5321
Chavis C, de Gourcy C, Dumont F, Imbach J-L (1983) NMR Studies of D-ribosylamines in solution: derivatives of primary amines. Carbohydr Res 113:1–20
Elad D (1976) Photochemistry of purines. In: Wang SY (ed) Photochemistry and photobiology of nucleic acids: vol I, chemistry. Academic Press, New York, pp 357–380
Ferris JP, Williams EA, Nicodem DE, Hubbard JS, Voecks GE (1974) Photolysis of CO-NH3 mixtures and the Martian atmosphere. Nature 249:437–439
Fina NJ, Edwards JO (1973) The alpha effect. A review. Int J Chem Kinetics 5:1–26
Folsome CE, Brittain A, Smith A, Chang S (1981) Hydrazines and carbohydrazides produced from oxidized carbon in Earth's primitive environment. Nature 294:64–65
Fuller WD, Sanchez RA, Orgel LE (1972a) Studies is prebiotic synthesis VI: synthesis of purine nucleosides. J Mol Biol 67:25–33
Fuller WD, Sanchez RA, Orgel LE (1972b) Studies is prebiotic synthesis VII: solid-state synthesis of purine nucleosides. J Mol Evol 1:249–257
Gonzales MA, Requejo JLJ, Albarran JCP, Perez JAG (1986) Facile preparation of C-glycosylbarbiturates and C-glycosylbarbituric acids. Carbohydr Res 158:53–66
Grekov AP, Veselov VYa (1978) The α-effect in the chemistry of organic compounds. Russ Chem Rev 47:631–648
Hammett LP (1940) Physical organic chemistry: reaction rates, equilibria, and mechanisms, 1st ed. McGraw-Hill, New York, pp 338–341
Jones AJ, Grant DM, Winkley MW, Robins RK (1970) Carbon-13 magnetic resonance. XVII1. Selected nucleosides. J Phys Chem 74: 2684–2689
Joyce GF (1987) Nonenzymatic template-directed synthesis of informational macromolecules. Cold Spring Harbor Symp Quant Biol 52:41–51
Joyce GF, Schwartz AW, Miller SL, Orgel LE (1987) The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc Natl Acad Sci USA 84:4398–4402
Komura H, Nakanishi K, Potvin BW, Stern HJ, Krooth RS (1980) Orotidine 5′-monophosphate decarboxylase inhibitors formed by spontaneous reaction of barbituric acid and ribose 5-phosphate, a surprising reaction. J Am Chem Soc 102:1208–1210
Miller SL, Orgel LE (1974) The origins of life on the Earth. Prentice-Hall, Englewood Cliffs, NJ, p 159
Pellizzari G (1891) Ricerche sulla guanidina. II. Fenilguanazolo. Gazz Chim Ital 2111:141–154
Pellizzari G (1894) Guanazolo e suoi derivati alchilici. Gazz Chin Ital 241:481–511
Piccirilli JA, Krauch T, Moroney SE, Benner SA (1990) Enzymatic incorporation of α new base pair into DNA and RNA extends the genetic alphabet. Nature 343:33–37
Pitsch S, Wendeborn S, Jaun B, Eschenmoser A (1993) Why pentoseand not hexose-nucleic acids? Part VII. Pyranosyl-RNA (“pRNA”). Helv Chim Acta 76:2161–2183
Pinner A (1887) Einwirkung von Harnstoff auf Phenylhydrazin. Chem Ber 20:2358–2360
Pontis H, Degerstedt G, Reichard P (1961) Uridine and deoxyuridine phosphorylases from Ehrlich ascited tumor. Biochim Biophys Acta 51:138–147
Robertson MP, Dworkin JP, Miller SL (1993) Prebiotic synthesis of hydroxymethyluracil and some t-RNA bases. Manuscript in preparation
Schmidt EW (1984) Hydrazine and its derivatives: preparation, properties, applications. John Wiley and Sons, New York, pp 36–48
Shapiro R (1988) Prebiotic ribose synthesis: a critical analysis. Orig Life Evol Biosph 18:71–85
Shoup ER, Miles HT, Becker ED (1966) NMR evidence of specific base-pairing between purines and pyrimidines. Biochim Biophys Res Comm 23:194–201
Szilágyi L, Györgydeák Z, Duddeck H (1986) Aldose-aminoguanidine condensation products: syntheses and N.M.R. studies. Carbohydr Res 158:67–79
Thiele J, Stange O (1894) Ueber Semicarbazid. Liebigs Ann Chem 283:1–46
Thornton JD, Spedding PL (1967) Hydrazine synthesis in the silent electrical discharge. Nature 213:1118–1119
Williams JM (1983) Tautomerism of saccharide hydrazones in solution and their reaction with nitrous acid. Carbohydr Res 117:89–94
Witkowski JT, Robins RK (1970) The chemical synthesis of the 1,2,4-triazole nucleosides related to uridine, 2′-deoxyuridine, thymidine, and cytidine. J Org Chem 35:2635–2641
Author information
Authors and Affiliations
Additional information
Correspondence to: S.L. Miller 0805
Rights and permissions
About this article
Cite this article
Kolb, V., Dworkin, J. & Miller, S. Alternative bases in the RNA world: The prebiotic synthesis of urazole and its ribosides. J Mol Evol 38, 549–557 (1994). https://doi.org/10.1007/BF00175873
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00175873