Summary
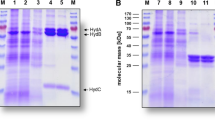
The benzoyl-CoA ligase from an anaerobic syntrophic culture was purified to homogeneity. It had a molecular mass of around 420 kDa and consisted of seven or eight subunits of 58 kDa. The temperature optimum was 37–40° C, the optimum pH around 8.0 and optimal activity required 50–100 mM TRIS-HCI buffer, pH 8.0 and 3–7 mM MgCl2; MgCl2 in excess of 10 mM was inhibitory. The activation energy for benzoate was 11.3 kcal/mol. Although growth occured only with benzoate as a carbon source, the benzoyl-coenzyme A (CoA) ligase formed benzoyl-CoA esters with benzoate, 2-, 3- and 4-fluorobenzoate, picolinate, nicotinate and isonicotinate. Acetate was activated to acetyl-CoA by an acetyl-CoA synthetase. The K m values for benzoate, 2-, 3- and 4-fluorobenzoate were 0.04, 0.28, 1.48 and 0.32 mM, the V max values 1.05, 1.0, 0.7 and 0.98 units (U)/mg, respectively. For reduced CoA (CoA-SH) a K m of 0.17 mM and a V max of 1.05 U/mg and for ATP a K m of 0.16 mM and a V max of 1.08 U/mg was determined. Benzoate activation was inhibited by more than 6 mM ATP, presumably by pyrophosphate generation from ATP. The inhibition constant (K i) for pyrophosphate was 5.7 mM. No homology of the N-terminal amino acid sequence with that of a 2-aminobenzoyl-CoA ligase of a denitrifying Pseudomonas sp. was found.
Similar content being viewed by others
References
Altenschmidt U, Fuchs G (1992) Novel aerobic 2-aminobenzoate metabolism. Purification and characterization of 2-aminobenzoate-CoA ligase, localization of the gene on a 8-kbp plasmid, and cloning and sequencing of the gene from a denitrifying Pseudomonas sp. Eur J Biochem 205: 721–727
Aranki A, Freter R (1972) Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. J Am Clin Nutr 25: 1329–1334
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43: 260–296
Bradford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Dangel W, Brackmann R, Lack A, Mohamed M, Koch J, Oswald B, Seyfried B, Tschech A, Fuchs G (1991) Differential expression of enzyme activities initiating anoxic metabolism of various aromatic compounds via benzoyl-CoA. Arch Microbiol 155: 256–262
Gallert C, Winter J (1992) Comparison of 4-hydroxybenzoate decarboxylase and phenol carboxylase activities in cell-free extracts of a defined, 4-hydroxybenzoate and phenol-degrading anaerobic consortium. Appl Microbiol Biotechnol 37: 119–124
Gallert C, Knoll G, Winter J (1991) Anaerobic carboxylation of phenol to benzoate: use of deuterated phenols revealed carboxylation exclusively in the C4-position. Appl Microbiol Biotechnol 36: 124–129
Geissler JF, Harwood CS, Gibson J (1988) Purification and properties of benzoate-coenzyme A ligase, a Rhodopseudomonas palustris enzyme involved in the anaerobic degradation of benzoate. J Bacteriol 170: 1709–1714
Knoll G, Winter J (1987) Anaerobic degradation of phenol in sewage sludge. Benzoate formation from phenol and CO2 in the presence of hydrogen. Appl Microbiol Biotechnol 25: 384–391
Knoll G, Winter J (1989) Degradation of phenol via carboxylation to benzoate by a defined, obligate syntrophic consortium of anaerobic bacteria. Appl Microbiol Biotechnol 30: 318–324
Lack A, Tommasi I, Aresta M, Fuchs G (1991) Catalytic properties of phenol carboxylase. In vitro study of CO2:4-hydroxybenzoate isotope exchange reaction. Eur J Biochem 197: 473–479
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Martinez-Blanco H, Reglero A, Rodriguez-Aparicio LB, Luengo JM (1990) Purification and biochemical characterization of phenylacetyl-CoA ligase from Pseudomonas putida. J Biol Chem 265: 7084–7090
Schultes V, Deutzmann R, Jaenicke R (1990) Complete amino-acid sequence of glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima. Eur J Biochem 192: 25–31
Sharak Genthner BR, Townsend GT, Chapman PJ (1989) Anaerobic transformation of phenol to benzoate via para-carboxylation: use of fluorinated analogues to elucidate the mechanism of transformation. Biochem Biophys Res Commun 162: 945–951
Tschech A, Fuchs G (1989) Anaerobic degradation of phenol via carboxylation to 4-hydroxybenzoate: in vitro studies of isotope exchange between 14CO2 and 4-hydroxybenzoate. Arch Microbiol 152: 594–599
Tschech A, Pfennig N (1984) Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol 137: 163–167
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterisation of the filamenteous gliding Desulfonema limicola gen. nov. sp. nov. and Desulfonema magnum sp. nov. Arch Microbiol 134: 286–294
Zellner G, Messner P, Kneifel H, Winter J (1989) Desulfovibrio simplex spec. nov., a new sulfate-reducing bacterium from a sour whey digester. Arch Microbiol 152: 329–334
Zhang X, Morgan TV, Wiegel J (1990) Conversion of 13C-1-phenol to 13C-4-benzoate, an intermediate step in the anaerobic degradation of chlorophenols. FEMS Microbiol Lett 67: 63–66
Ziegler K, Braun K, Böckler A, Fuchs G (1987) Studies on the anaerobic degradation of benzoic acid 2-aminobenzoic acid by a denitrifying Pseudomonas strain. Arch Microbiol 149: 62–69
Ziegler K, Buder R, Winter J, Fuchs G (1989) Activation of aromatic acids and aerobic 2-aminobenzoate metabolism in a denitrifying Pseudomonas strain. Arch Microbiol 151: 171–176
Author information
Authors and Affiliations
Additional information
Correspondence to: J. Winter
Rights and permissions
About this article
Cite this article
Auburger, G., Winter, J. Purification and characterization of benzoyl-CoA ligase from a syntrophic, benzoate-degrading, anaerobic mixed culture. Appl Microbiol Biotechnol 37, 789–795 (1992). https://doi.org/10.1007/BF00174847
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174847