Summary
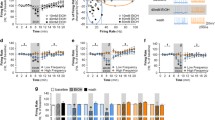
Fast cyclic voltammetry using carbon fibre microelectrodes in rat brain slices, was used to investigate regional differences in electrically-evoked dopamine (DA) efflux at 10 different sites in the anterior caudate putamen (aCPu) and 10 sites in the posterior caudate putamen (pCPu). For each site DA overflow was evoked by both single pulse (1P) stimulation and by trains of 25 pulses applied at a frequency of 50 Hz (25P/50 Hz). Peak DA efflux evoked by 1P was about 58% greater in the aCPu (0.19 μmol/l DA) than in the pCPu (0.12 μmol/l DA), but showed no mediolateral variation in either region. Peak DA efflux evoked by 25P/50 Hz relative to 1P efflux also varied between the two regions; the aCPu contained predominantly low ratio (25P/50 HZ: 1P) sites ranging from 1.47 to 3.71, whereas in the pCPu these ratios were higher, ranging from 2.73 to 9.40, and were particularly high in the dorsomedial region of the pCPu. Efflux detected in low ratio sites of the aCPu showed little dependence on the frequency (10 to 500 Hz), or the number of pulses (5 to 20) in a train. By contrast DA efflux evoked in high ratio sites of the pCPu responded in a pulse and frequency dependent manner, the maximum ratio (approximately 8 times 1P) being at 20P/20 Hz. Interestingly the frequency response relationship obtained in the pCPu resembled the profile observed in the nucleus accumbens (NAc).
Voltammetric evidence and experiments with selective reuptake blockers indicated that only DA was measured in our studies and 5-HT did not significantly contribute to the frequency dependent pattern of efflux detected in high ratio sites of the pCPu, where striatal 5-HT concentrations are highest. Experiments with the selective D2 receptor antagonists metoclopramide or (−)sulpiride revealed that under our experimental conditions, DA efflux in the aCPu was not modulated by DA autoreceptor activation. By contrast, autoreceptor modulation did occur in high ratio sites of the pCPu at stimulations lasting longer than approximately 1000 ms.
These observations support the concept that the caudate putamen is heterogeneously organised with respect to the frequency characteristics of evoked DA release. The factors controlling frequency dependent release under these conditions may be a function of A10 innervation, since high ratio release sites occur in areas where the density of such innervation is greatest, for example, the dorsomedial pCPu. This is supported by the observation that high ratio release sites are also found in the NAc, which receives dopaminergic fibres predominantly from an A10 region. However, the involvement of different regionally distributed transmitters acting on presynaptic receptors involved in the regulation of dopamine release, or differences between nerve terminals in striosomes and matrix, cannot be excluded.
Similar content being viewed by others
References
Beal MF, Martin JB (1985) Topographical dopamine and serotonin distribution and turnover in rat striatum. Brain Res 358:10–15
Bull DR, Sheehan MJ (1991) Presynaptic regulation of electrically evoked dopamine overflow in nucleus accumbens: a pharmacological study using fast cyclic voltammetry in vitro. Naunyn-Schmiedeberg's Arch Pharmacol 343:260–265
Bull DR, Palij P, Sheehan MJ, Millar J, Stamford JA, Kruk ZL, Humphrey PPA (1990) Application of fast cyclic voltammetry to measurement of electrically evoked dopamine overflow from brain slices in vitro. J Neurosci Methods 32:37–44
Chiodo LA, Bunney BS (1983) Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci 3:1607–1619
Freeman AS, Meltzer LT, Bunney BS (1985) Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci 36:1983–1994
Gerfen CR, Herkenham M, Thibault J (1987) The neostriatal mosaic. II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci 7:3915–3934
Glynn GE, Yamamoto BK (1989) In vivo neurochemical and anatomical heterogeneity of the dopamine uptake system in the rat caudate putamen. Brain Res 481:235–241
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. 1. Identification and characterization. Neuroscience 10:301–315
Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4:2866–2876
Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254
Graybiel AM, Ragsdale CW Jr (1978) Histochemically distinct compartments in the striatum of human, monkey and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA 75:5723–5726
Jimenez-Castellanos J, Graybiel AM (1987) Subdivisions of the dopamine — containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience 23:223–242
Kemel M-L, Desban M, Glowinski J, Gauchy C (1989) Distinct presynaptic control of dopamine release in striosomal and matrix areas of the cat caudate nucleus. Proc Natl Acad Sci USA 86:9006–9010
Krebs MO, Trovero F, Desban M, Gauchy C, Glowinski J, Kemel M-L (1991) Distinct presynaptic regulation of dopamine release through NMDA receptors in striosome- and matrix-enriched areas of the rat striatum. J Neurosci 11:1256–1262
Kuhr WG, Wightman RM (1986) Real-time measurement of dopamine release in rat brain. Brain Res 381:168–171
Limberger N, Trout SJ, Kruk ZL, Starke K (1991) “Real time” measurement of endogenous dopamine release during short trains of pulses in slices of rat neostriatum and nucleus accumbens: role of autoinhibition. Naunyn-Schmiedeberg's Arch Pharmacol 344:623–629
Millar J, Stamford JA, Kruk ZL, Wightman RM (1985) Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. Eur J Pharmacol 109:341–348
O'Connor JJ, Kruk ZL (1991 a) Fast cyclic voltammetry can be used to measure stimulated endogenous 5-hydroxytryptamine release in untreated rat brain slices. J Neurosci Methods 38:25–33
O'Connor JJ, Kruk ZL (1991 b) Frequency dependence of 5-HT autoreceptor function in rat dorsal raphe and suprachiasmatic nuclei studied using fast cyclic voltammetry. Brain Res 568:123–130
Palij P, Bull DR, Sheehan MJ, Millar J, Stamford JA, Kruk ZL, Humphrey PPA (1990) Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res 509:172–174
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Second edition. Academic Press, London
Richards CD, Tegg WJB (1977) A superfusion chamber suitable for maintaining mammalian brain tissue slices for electrical recording. Br J Pharmacol 59:526P
Stamford JA, Kruk ZL, Millar J (1986) Subsecond striatal dopamine release measured by in vivo voltammetry. Brain Res 381:351–355
Starke K, Göthert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69:864–989
Trout SJ, Kruk ZL (1991) Frequency dependent dopamine efflux from the nucleus accumbens in rat brain slices: The influence of autoreceptors. Br J Pharmacol 104:260P
Trout SJ, Kruk ZL (1992) Differences in evoked dopamine efflux in rat caudate putamen, nucleus accumbens and tuberculum olfactorium in the absence of uptake inhibition: influence of autoreceptors. Br J Pharmacol 106:452–458
Trout SJ, Patel J, Kruk ZL (1991) Characteristics of autoreceptor control of dopamine release in olfactory tubercle in the absence of uptake inhibition. Br J Pharmacol 102:9P
White FJ, Wang RY (1983) Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science 221:1054–1057
Widmann R, Sperk G (1986) Topographical distribution of amines and major amine metabolites in the rat striatum. Brain Res 367:244–249
Yamamoto BK, Pehek EA (1990) A neurochemical heterogeneity of the rat striatum measured by in vivo electrochemistry and microdialysis. Brain Res 506:236–242
Author information
Authors and Affiliations
Additional information
Send offprint requests to S. J. Trout at the above address
Rights and permissions
About this article
Cite this article
Patel, J., Trout, S.J. & Kruk, Z.L. Regional differences in evoked dopamine efflux in brain slices of rat anterior and posterior caudate putamen. Naunyn-Schmiedeberg's Arch Pharmacol 346, 267–276 (1992). https://doi.org/10.1007/BF00173539
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173539