Abstract
Extracellular α-glucosidase, β-glucosidase, and aminopeptidase activity variations (measured by use of fluorogenic substrate analogs) at a coastal station in the Mediterranean Sea were investigated over a 1-year period. A 27-h cycle and daily measurements were made in a summer situation. We observed strong relative diurnal variations, compared to seasonal variations, in α- and β-glucosidase. Within 24 h, 0–100% of both α- and β-glucosidase were found in the dissolved phase. The aminopeptidase activities did not show a strong diurnal variation, but day to day variations were similar in magnitude to seasonal changes. Consistently, high proportions of all three enzymes were found in the dissolved phase on a seasonal scale. Seasonal measurements at 50- and 100-m depths showed a weak negative dependency on depth for extracellular enzyme activity. The potential importance of both hourly and daily changes in extracellular enzyme activity and of free enzymes is considered.
Similar content being viewed by others
References
Albertson NH, Nyström T, Kjelleberg S (1990) Exoprotease activity of two marine bacteria during starvation. Appl Environ Microbiol 56:218–223
Azam F, Cho BC (1987) Bacterial utilization of organic matter in the sea. In: Ecology of microbial communities. Cambridge University Press, Cambridge, pp 261–281
Azam F, Fenchel T, Field JG, Gray IS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Azam F, Smith DC (1991) Bacterial influence on the variability in the ocean's biogeochemical state: a mechanistic view. In: Demers (ed) Particle analysis in oceanography. Springer, Berlin-Heidelberg, pp 213–236
Billen G, Fontigny A (1987) Dynamics of a Phaeocystis-dominated spring bloom in Belgian coastal waters. 11. Bacterioplankton dynamics. Mar Ecol Prog Ser 37:249–257
Burney CM (1986) Bacterial utilization of total in situ dissolved carbohydrate in offshore waters. Limnol Oceanogr 31(2):427–431
Cho BC, Azam F (1988) Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 332:441–443
Chróst RJ (1989) Characterization and significance of β-glucosidase activity in lake water. Limnol Oceanogr 34:660–672
Chróst RJ, Münster U, Rai H, Albrecht D, Witzel PK, Overbeck J (1989) Photosynthetic production and exoenzymatic degradation of organic matter in the euphotic zone of a eutrophic lake. J Plankton Res 11:223–242
Fontigny A, Billen G, Vives-Rego J (1987) Some kinetic characteristics of exoproteolytic activity in coastal seawater. Estuar Coast Shelf Sci 25:127–133
Fuhrman JA, Bell TM (1985) Biological considerations in the measurement of dissolved free amino acids in seawater and implications for chemical and microbiological studies. Mar Ecol Prog Ser 25:13–21
Fuhrman JA, Eppley RW Hagström A, Azam F (1985) Diel variations in bacterioplankton, phytoplankton, and related parameters in the Southern California Bight. Mar Ecol Prog Ser 27: 9–20
González JM, Sherr BF, Sherr EB (1993) Digestive enzyme activity as a quantitative measure of protistan grazing: the acid lysozyme assay for bacterivory. Mar Ecol Prog Ser 100:197–206
Halemejko GZ, Chróst RJ (1986) Enzymatic hydrolysis of proteinaceous particulate and dissolved material in an eutrophic lake. Arch Hydrobiol 107:1–21
Hollibaugh JT, Azam F (1983) Microbial degradation of dissolved proteins in seawater. Limnol Oceanogr 28:1104–1116
Hoppe H-G (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Hoppe H-G (1986) Relations between bacterial extracellular enzyme activities and heterotrophic substrate uptake in a brackish water environment. In: Gerbam Deuxième Colloque International de Bactériologie Marine. IFREMER, Actes de Colloques, 3, pp 119–128
Hoppe HG (1991) Microbial extracellular enzyme activity: anew key parameter in aquatic ecology. In: Chróst RJ (ed) Microbial ectoenzymes in aquatic environments. Springer-Verlag, New York, pp 60–82
Hoppe H-G, Kim S-J, Gocke K(1988) Microbial decomposition in aquatic environments: combined process of extracellular enzyme activity and substrate uptake. Appl Environ Microbiol 54:784–790
Hoppe H-G, Schramm W Bacolod P (1988) Spatial and temporal distribution of pelagic microorganisms and their proteolytic activity over a partly destroyed coral reef. Mar Ecol Prog Ser 44:95–102
Kaener M, Ferrier-Pagés C, Rassoulzadegan F (1994) Phagotrophic nanoflagellates contribute to occurrence of α-glucosidase and aminopeptidase in marine environments. Mar Ecol Prog Ser 114:237–244
Kamer M, Fuks D, Hemdl GJ (1992) Bacterial activity along a trophic gradient. Microb Ecol 24:243–257
Kamer M, Herndl GJ (1992) Extracellular enzymatic activity and secondary production in free-living and marine-snow-associated bacteria. Mar Biol 113:341–347
Kendall MG, Stuart A (1963–1968) The advanced theory of statistics. Griffin, London
Lancelot C, Billen G (1984) Activity of heterotrophic bacteria and its coupling to primary production during the spring phytoplankton bloom in the southern bight of the North Sea. Limnol Oceanogr 29:721–730
Mague TH, Friberg E, Hughes DJ, Morris I (1980) Extracellular release of carbon by marine phytoplankton; a physiological approach. Limnol Oceanogr 25:262–279
Mayer LM (1989) Extracellular proteolytic enzyme activity in sediments of an intertidal mudflat. Limnol Oceanogr 34:973–981
Nagata T, Kirchman DL (1990) Filtration-induced release of dissolved free amino acids: application to cultures of marine protozoa. Mar Ecol Prog Ser 68:1–5
Nagata T, Kirchman DL (1992) Release of macromolecular organic complexes by heterotrophic marine flagellates. Mar Ecol Prog Ser 83:233–240
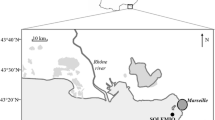
Nival P, Corre MC (1976) Variation annuelle des charactéristiques hydrologiques de surface dans la rade de Villefranche-sur-mer. Ann Inst Océanogr 52:57–78
Porter K, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62
Rath J, Schiller C, Herndl GJ (1993) Ectoenzymatic activity and bacterial dynamics along a trophic gradient in the Caribbean Sea. Mar Ecol Prog Ser 102:89–96
Riemann B, Bjørnsen PK (1987) Calculation of cell production of coastal marine bacteria based on measured incorporation of 3H thymidine. Limnol Oceanogr 32(2):471–476
Sokal RR, Rohlf FJ (1981) Biometry. Freeman & Co, New York
Somville M (1984) Measurement and study of substrate specificity of exoglucosidase activity in eutrophic water. Appl Environ Microbiol 48:1181–1185
Vives-Rego J, Billen G, Fontigny A, Somville M 0(1985) Free and attached proteolytic activity in water environments. Mar Ecol Prog Ser 21:245–249
Vrba J, Simek K, Nedoma J, Hartman P (1993) 4-methylumbelliferyl-β-N-acetylglucosaminide hydrolysis by a high-affinity enzyme, a putative marker of protozoan bacterivory. Appl Environ Microbiol 59(9):3091–3101
Ward BB (1984) Photosynthesis and bacterial utilization of phytoplankton exudates: results from pre- and post-incubation size fractionation. Oceanol Acta 7:337–343
Wheeler JR (1976) Fractionation by molecular weight of organic substances in Georgia coastal water. Limnol Oceanogr 21:846–852
Author information
Authors and Affiliations
Additional information
Correspondence to: M. Karner
Rights and permissions
About this article
Cite this article
Kamer, M., Rassoulzadegan, F. Extracellular enzyme activity: Indications for high short-term variability in a coastal marine ecosystem. Microb Ecol 30, 143–156 (1995). https://doi.org/10.1007/BF00172570
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00172570