Summary
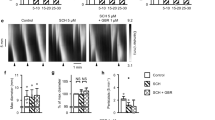
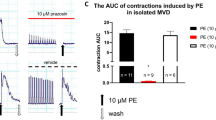
The effect of octopamine on intestinal smooth muscle of rabbit isolated jejunum has been studied. Octopamine induced a dose-dependent decrease of muscle tone and this reproducible relaxation was not modified by tetrodotoxin or by agents that acted on adrenergic nerve terminals. Adrenoceptor antagonists, at concentrations sufficient to block each adrenoceptor type, did not reduce the actions of octopamine. On the other hand, octopamineinduced relaxations were affected by agents that have the ability to change cyclic AMP (cAMP) content; such as alloxan (an adenylate cyclase inhibitor), imidazole (a stimulator of phosphodiesterase), and isobutyl methylxanthine (an inhibitor of phosphodiesterase). Direct stimulation of adenylate cyclase by octopamine was demonstrated using radioimmunoassay of cAMP. Furthermore, haloperidol and perphenazine at concentration required to block dopamine receptor sites attenuated both smooth muscle relaxation and the formation of cAMP induced by octopamine. The effect of octopamine was totally blocked by SCH 23390, an antagonist of dopamine D-1 receptors. The lack of effect of domperidone and sulpiride, antagonists of dopamine D-2 receptors, on the actions of octopamine excludes the involvement of dopamine D-2 receptors. These results suggest that octopamine acts on intestinal dopamine D-1 receptor sites to produce relaxation of rabbit jejunum through an increase of cAMP.
Similar content being viewed by others
References
Albano JDM, Mudsley DV, Brown BL, Barnes GD (1973) A simplified procedure for the determination of adenylate cyclase activity. Biochem Soc Trans 1:477–479
Axelrod J, Saavedra JM (1977) Octopamine. Nature 265:501–504
Bass AS, Robie NW (1984) Stereoselectivity of S- and R-sulpiride for pre- and postsynaptic dopamine receptors in the canine kidney. J Pharmacol Exp Ther 229:67–71
Cavero I, Massingham R, Lefevre-Borg F (1982) Peripheral dopamine receptors, potential targets for a new class of antihypertensive agents. Part I: subclassification and functional description. Life Sci 31:939–948
Cheng JT, Shen CL (1986) Tyramine-induced release of neuropeptide Y (NPY) in isolated rabbit intestine. Eur J Pharmacol 123:303–306
Cheng JT, Tuan YH, Shen CL (1987) Characterization of the release of neuropeptide Y (NPY) induced by tyramine from synaptosomal preparations of rabbit jejunum. Eur J Pharmacol 136:23–30
Cohen KL, Bitensky MW (1969) Inhibitory effects of alloxan on mammalian adenyl cyclase. J Pharmacol Exp Ther169:80–86
Connor JD (1984) Antipsychotic and antianxiety drugs. In: Goth A (ed) Goth medical pharmacology, 11th edn. Mosby, St. Louis, pp 251–259
Danielson TJ, Boulton AA, Robertson HA (1977) m-Octopamine, p-octopamine and phenylethanolamine in rat brain: a sensitive, specific assay and the effects of drugs. J Neurochem 29:1131–1135
David JC, Coulon JF (1985) Octopamine in invertebrates and vertebrates: a review. Prog Neurobiol 24:141–185
Delacour J, Coulon JF, David JC, Guenaine C (1983) Brain octopamine and the strain differences in avoidance behavior. Brain Res 288:169–176
Duffield PH, Dougan DFH, Wade DN, Duffield AM (1981) A chemical ionization gas chromatographic mass spectrometric assay for octopamine and tyramine in rat brain. Biomed Mass Spectrom 8:170–173
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1123
Gershon MD (1967) Effects of tetrodotoxin on innervated smooth muscle preparations. Br J Pharmacol 29:259–279
Harmar AJ, Horn AS (1976) Octopamine in mammalian brain: rapid post mortem increase and effects of drugs. J Neurochem 26:987–993
Heilman RD, Lum BkB (1971) Studies on the intestinal relaxation produced by dopamine. J Pharmacol Exp Ther 178:63–72
Helfman DM, Kuo JF (1982) Differential effects of various phosphodiesterase inhibitors, pyrimidine and purine compounds, and inorganic phosphate on cyclic CMP, cyclic AMP and cyclic GMP phosphodiesterase. Biochem Pharmacol 31:43–47
Hicks TP (1977) The possible role of octopamine as a synaptic transmitter: a review. Can J Physiol Pharmacol 55:137–152
Hyttel J (1983) SCH23390 — the first selective dopamine D-1 antagonist. Eur J Pharmacol 91:153–154
Ilhan M, Long JP, Cannon JG (1976) Effects of some dopamine analogs and haloperidol on response to stimulation of adrenergic nerves using cat atria in vitro. Arch Int Pharmacodyn 219:193–204
Kao CY (1966) Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev 18:997–1049
Katz B, Miledi R (1967) Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond [Biol] 167:8–22
Kebabian JW, Calne DB (1979) Multiple receptors for dopamine. Nature 277:93–96
Kohli JD, Glock D, Goldberg LI (1983) Selective DA2 versus DA1 antagonist activity of domperidone in the periphery. Eur J Pharmacol 89:137–141
Korol B, Soffer L, Brown ML (1968) Some cardiovascular studies on octopamine. Arch Int Pharmacodyn Ther 171:415–424
Kupfermann I (1980) Role of cyclic nucleotides in excitable cells. Annu Rev Physiol 42:629–641
Lafon-Cazal M, Bockaert J (1985) Pharmacological characterization of octopamine-sensitive adenylate cyclase in the flight muscle of Locusta Migratoria L. Eur J Pharmacol 119:53–59
Lowry OH, Rosebrough NY, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Molinoff P, Axelrod J (1969) Octopamine: normal occurrence in sympathetic nerves of rats. Science 164:428–429
Raiteri M, Del Carmine R, Bertollini A, Levi G (1977) Effect of desmethylimipramine on the release of 3H-norepinephrine induced by various agent in hypothalamic synaptosomes. Mol Pharmacol 13:746–758
Robertson HA, Juorio AV (1976) Octopamine and some related non-catecholic amines in invertebrate nervous system. Annu Rev Neurobiol 19:173–224
Sandler M, Ruthven CRJ, Goodwin BL, Reynolds GP, Rao VAR, Coppen A (1979) Deficient production of tyramine and octopamine in cases of depression. Nature 278:357–358
Sokal R, Rohlf FJ (1969) Single classification analysis of variance. In: Emerson R, Kennedy D, Park RB (eds) Biometry, Freeman, San Francisco, pp 204–252
Starke K (1972) Interactions of guanethidine and indirect-acting sympathomimetic amines. Arch Int Pharmacodyn Ther 195: 309–314
Steiner AL, Pagliara AS, Chase LR, Kipnis DM (1982) Radioimmunoassay for cyclic nucleotides. 11. Adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in mammalian tissues and body fluids. J Biol Chem 257:1114–1120
Van Rossum JM (1963) Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and evaluation of drug parameters. Arch Int Pharmacodyn Ther 143:299–330
Williams CM, Couch MW, Thonoor CM, Midgley JM (1987) Isomeric octopamines: their occurrence and functions. J Pharm Pharmacol 39:153–157
Author information
Authors and Affiliations
Additional information
Send offprint requests to J. T. Cheng at the above address
Rights and permissions
About this article
Cite this article
Cheng, JT., Hsieh-Chen, SC. Octopamine relaxes rabbit jejunal smooth muscle by selective activation of dopamine D1 receptors. Naunyn-Schmiedeberg's Arch Pharmacol 338, 373–378 (1988). https://doi.org/10.1007/BF00172112
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00172112