Abstract
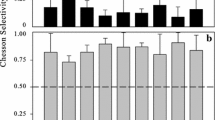
Seasonal and depth variations of the abundance, biomass, and bacterivory of protozoa (heterotrophic and mixotrophic flagellates and ciliates) were determined during thermal stratification in an oligomesotrophic lake (Lake Pavin, France). Maximal densities of heterotrophic flagellates (1.9 × 103 cells ml−1) and ciliates (6.1 cells ml−1) were found in the metalimnion. Pigmented flagellates dominated the flagellate biomass in the euphotic zone. Community composition of ciliated protists varied greatly with depth, and both the abundance and biomass of ciliates was dominated by oligotrichs. Heterotrophic flagellates dominated grazing, accounting for 84% of total protistan bacterivory. Maximal grazing impact of heterotrophic flagellates was 18.9 × 106 bacteria 1−1h−1. On average, 62% of nonpigmented flagellates were found to ingest particles. Ciliates and mixotrophic flagellates averaged 13% and 3% of protistan bacterivory, respectively. Attached protozoa (ciliates and flagellates) were found to colonize the diatom Asterionella formosa. Attached bacterivores had higher ingestion rates than free bacterivorous protozoa and may account for 66% of total protozoa bacterivory. Our results indicated that even in low numbers, epibiotic protozoa may have a major grazing impact on free bacteria.
Similar content being viewed by others
References
Amblard C (1988) Seasonal succession and strategies of phytoplankton development in two lakes of different trophic states. J Plankton Res 10:189–1208
Amblard C, Carrias J-F, Bourdier G, Maurin N (1994) The microbial loop in a humic lake: seasonal and vertical variations in the structure of the different communities. Hydrobiologia 300/301:71–84
Amblard C, Sime-Ngando T, Rachiq S, Bourdier G (1993) Importance of ciliated protozoa in relation to the bacterial and phytoplanktonic biomass in an oligo-mesotrophic lake during the spring diatom bloom. Aquat Sci 55/1:1–9
Beaver JR, Crisman TL (1982) The trophic response of ciliated protozoans in freshwater lakes. Limnol Oceanogr 27:246–253
Bennett S-J, Sanders RW Porter KG (1990) Heterotrophic, autotrophic, and mixotrophic nanoflagellates: seasonal abundances and bacterivory in a eutrophic lake. Limnol Oceangr 35:1821–1832
Berman T, Nawrocki M, Taylor GT, Karl DM (1987) Nutrient flux between bacteria, bacterivorous nanoplanktonic protists, and algae. Mar Microb Food Webs 2:69–82
Bird DF, Kalff J (1986) Bacterial grazing by planktonic algae. Science 231:493–495
Bloem JF, Ellenbroek M, Bar-gilissen M-JB, Cappenberg TE (1989) Protozoan grazing and bacterial production in stratified Lake Vechten estimated with fluorescently labeled bacteria and thymidine incorporation. Appl Environ Microbiol 55:1787–1795
Børsheim KY, Bratbak G (1987) Cell volume to cell carbon conversion factors for a bacteriovorous Monas sp. enriched from seawater. Mar Ecol Prog Ser 36:171–175
Caron DA (1983) Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol 46:491–498
Caron DA (1987) Grazing of attached bacteria by heterotrophic microflagellates. Microb Ecol 13:203–217
Caron DA, Goldman JC, Dennett MR (1988) Experimental demonstration of the roles of bacteria and bacterivorous protozoa in plankton nutrient cycles. Hydrobiologia 159:27–40
Caron DA, Pick FR, Lean LDR (1985) Chroococcoid cyanobacteria in Lake Ontario: vertical and seasonal distribution during 1982. J Phycol 21:171–175
Carrias J-F, Amblard C, Bourdier G (1994) Vertical and temporal heterogeneity of planktonic ciliated protozoa in a humic lake. J Plankton Res 16:471–485
Carrick HJ, Fahnenstiel GL (1989) Biomass, size structure, and composition of phototrophic and heterotrophic nanoflagellate communities in Lakes Huron and Michigan. Can J Fish Aquat Sci 46:1922–1928
Carrick HJ, Fahnenstiel GL (1990) Planktonic protozoa in Lakes Huron and Michigan: seasonal abundance and composition of ciliates and dinoflagellates. J Great Lakes Res 16:319–329
Carrick HJ, Fahnenstiel GL, Stoermer EF, Wetzel RG (1991) The importance of zooplankton-protozoan trophic coupling in Lake Michigan. Limnol Oceanogr 36:1335–1345
Corliss JO (1979) The ciliates protozoa characterization, classification, and guide to the literature, 2nd edn. Pergamon Press, New York
Cynar FJ, McN. Sieburth J (1986) Unambiguous detection and improved quantification of phagotrophy in apochloric nanoflagellates using fluorescent microspheres and concomitant phase contrast and epifluorescence microscopy. Mar Ecol Prog Ser 32:61–70
Fahnenstiel GL, Carrick HJ, Iturriaga R (1991) Physiological characteristics and foodweb dynamics of Synechococcus in Lakes Huron and Michigan. Limnol Oceanogr 36:219–234
Fenchel T (1982) Adaptations to heterogenous environments. (Ecology of heterotrophic microflagellates, vol 3) Mar Ecol Prog Ser 9:25–33
Fenchel T (1986) The ecology of heterotrophic microflagellates. Adv Microbial Ecol 9:57–97
Foissner VW, Oleksiv I, Müller H (1989) Morphology and infraciliature of some ciliates (Protozoa, Ciliophora) from stagnant waters. Arch Protistenkd 138:191–206
Foissner W (1994) Progress in taxonomy of planktonic freshwater ciliates. Mar Microb Food Webs 8:9–35
Hall JA, Barrett DP, James RJ (1993) The importance of phytoflagellate, heterotrophic flagellate, and ciliate grazing on bacteria and picophytoplankton-sized prey in a coastal marine environment. J Plankton Res 15:1075–1086
Jones RI, Rees S (1994) Characteristics of particle uptake by the phagotrophic phytoflagellate, Dinobryon divergens. Mar Microb Food Webs 8:97–110
Jonsson PR (1986) Particle size selection, feeding rates, and growth dynamics of marine planktonic oligotrichous ciliates (Ciliophora, Oligotrichida). Mar Ecol Prog Ser 33:265–277
Jonsson PR (1987) Photosynthetic assimilation of inorganic carbon in marine oligotrich ciliates (ciliophora, oligotrichina). Mar Microb Food Webs 2:55–68
Jürgens K (1994) Impact of daphnia on planktonic microbial food webs: a review. Mar Microb Food Webs 8:295–324
Kahl A (1930–1935) Urtiere order Protozoa. 1: Wimpertier oder Cilata (Infusoria), eine bearbeitung der freilebenden und ectocommensalen Infusorien der Erde, unter Ausschluss der marines Tintinnidae. In: Dahl F (ed) Die Tierwelt Demschlands. G. Fisher, Jena, parts 18 (year 1930), 21 (1931), 25 (1932), 30 (1935)
Komarek J (1976) Taxonomic review of the genera Synechocystis Sauv. 1892, Synechococcus Näg. 1849, and Cyanothece gen. nov. (Cyanophyceae). Arch Protistenkd 118:119–179
Kudo R (1966) Protozoology. Charles C. Thomas, Springfield, Illinois
Laval-Peuto M, Rassoulzadegan F (1988) Autofluorescence of marine planktonic Oligotrichina and other ciliates. Hydrobiologia 159:99–110
Laybourn-Parry J, Olver J, Rogerson A, Duverge PL (1990) The temporal and spatial patterns of protozoa plankton abundance in a eutrophic temperate lake. Hydrobiologia 203:99–110
Marchant HJ, Scott FJ (1993) Uptake of submicrometer particles and dissolved organic material by antartic choanoflagellates. Mar Ecol Prog Ser 92:59–64
McManus GB, Fuhrman JA (1988) Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia 159:51–62
McManus GB, Okubo A (1991) On the use of surrogate food particles to measure protistan ingestion. Limnol Oceanogr 36:613–617
Mitchell GC, Baker JH, Sleigh MA (1988) Feeding of a freshwater flagellate, Bodo saltans, on diverse bacteria. J Protozool 35:219–222
Miller H (1989) The relative importance of different ciliate taxa in the pelagic food web of Lake Constance. Microb Ecol 18:261–273
Nagata T (1988) The microflagellate picoplankton food linkage in the water column of Lake Biwa. Limnol Oceanogr 33:504–517
Pace ML (1982) Planktonic ciliates: their distribution, abundance, and relationship to microbial resources in a monomictic lake. Can J Fish Aquat Sci 39:1106–116
Pace ML, Bailif MD (1987) Evaluation of a fluorescent microsphere technique for measuring grazing rates of phagotrophic microorganisms. Mar Ecol Prog Ser 40:185–193
Pace ML, McManus GB, Findlay SEG (1990) Planktonic community structure determines the fate of bacterial production in a temperate lake. Limnol Oceanogr 35:795–808
Pick FR, Caron DA (1987) Picoplankton and nanoplankton biomass in Lake Ontario: relative contribution of phototrophic and heterotrophic communities. Can J Fish Aquat Sci 44:2164–2172
Porter KJ, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Putt M, Stoecker DK (1989) An experimentally determined carbon:volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnol Oceanogr 34:1097–1103
Riemann B, Christoffersen K (1993) Microbial trophodynamics in temperate lakes. Mar Microb Food Webs 7:69–100
Sanders RW, Porter KG (1987) Phagotrophic phytoflagellates. Adv Microb Ecol 10:167–192
Sanders RW, Porter KG, Bennett SJ, Debiase AE (1989) Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol Oceanogr 34:673–687
Sherr BF Sherr EB (1987) Role of heterotrophic protozoa in carbon and energy flow in aquatic ecosystems. In: Klug MJ, Reddy CA (eds), Current perspectives in microbial ecology. Amer Soc Microbiol, Washington DC, pp 412–423
Sherr BF, Sherr EB, Fallon RD (1987) Use of monodispersed, fluorescently-labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol 53:958–965
Sherr EB (1988) Direct use of high molecular weight polysaccharide by heterotrophic flagellates. Nature 335:1225–1227
Sherr EB, Sherr BF (1987) High rates of consumption of bacteria by pelagic ciliates. Nature 325:710–711
Sherr EB, Sherr BF, Berman T, Hadas O (1991) High abundance of picoplanktonic-ingesting ciliates during late fall in Lake Kinneret, Israel. J Plankton Res 13:789–799
Simek K, Straskrabova V (1992) Bacterioplankton production and protozoan bacterivory in a mesotrophic reservoir. J Plankton Res 14:773–787
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Sommaruga R, Psenner R (1993) Nanociliates of the order Prostomatida: Their relevance in the microbial food web of a mesotrophic lake. Aquat Sci 55:179–187
Stockner JG, Porter KG (1988) Microbial food webs in freshwater planktonic ecosystems. In: Carpenter SJ (ed), Complex interactions in lake communities. Springer, New York, pp 69–83
Stoecker DK, McDowell Capuzzo J (1990) Predation on protozoa: its importance to zooplankton. J Plankton Res 12:891–908
Stoecker DK, Taniguchi A, Michaels AE (1989) Abundance of autotrophic, mixotrophic, and heterotrophic planktonic ciliates in shelf and slope waters. Mar Ecol Prog Ser 50:241–254
Tranvik LJ, Porter KG, McN Sieburth J (1989) Occurrence of bacterivory in Cryptomonas, a common freshwater phytoplankoor. Oecologia 78:473–476
Tsuji T, Ohki K, Fujita K (1986) Determination of photosynthetic pigment composition in an individual phytoplankton cell in seas and lakes using fluorescence microscopy: properties of the fluorescence emitted from picophytoplankton cells. Mar Biol 93:343–349
Utermöhl H (1958) Zur vervollkommnung der quantitative phytoplankton-Methodik. Mitt Int Ver Limnol 9:1–38
Vaqué D, Pace ML (1992) Grazing on bacteria by flagellates and cladocerans in lakes of contrasting food-web structure. J Plankton Res 14:307–321
Verity PG, Roberson CY, Tronzo CR, Andrews MG, Nelson JR, Sieracki ME (1992) Relationships between cell volume and the carbon and nitrogen content of marine photosynthetic nanoplankton. Limnol Oceanogr 37:1434–1446
Weisse T (1988) Dynamics of autotrophic picoplankton in Lake Constance. J Plankton Res 10:1179–1188
Author information
Authors and Affiliations
Additional information
Correspondence: C. Amblard.
Rights and permissions
About this article
Cite this article
Carrias, J.F., Amblard, C. & Bourdier, G. Protistan bacterivory in an oligomesotrophic lake: Importance of attached ciliates and flagellates. Microb Ecol 31, 249–268 (1996). https://doi.org/10.1007/BF00171570
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00171570