Summary
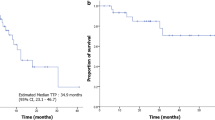
Etoposide (VP-16), 150 mg/M2, given intravenously daily for 3 days every 3 weeks resulted in 3 complete responses and 6 partial responses in 154 patients with a spectrum of recurrent malignant solid tumors. There was evidence of disease control in an additional 37 patients (27 mixed responses and 10 stable disease). These responses occurred primarily in patients with Ewing's sarcoma, Hodgkin's disease, neuroblastoma and rhabdomyosarcoma. Most of the patients had every extensive prior therapy; however prior therapy with teniposide (VM-26), the congener of VP-16, did not seem to preclude responses to the latter drug. Myelosuppression was the principal form of toxicity. Neutropenia characterized by absolute neutrophil counts of 0.5 to 0.9 × 109/L occurred in one-half of the patients, and thrombopenia with platelet counts of < 25 to 49 × 109/L in one-fourth. These results demonstrate a favorable therapeutic index for VP-16 in several recurrent childhood solid tumors, supporting its use as a component of primary therapy for these diseases.
Similar content being viewed by others
References
Keller-Juslen C, Kuhn M, Von Wartburg A, Stahelin H: Synthesis and antimitotic activity of glycosidic lignan derivatives related to podophyllotoxin. J Med Chem 14: 936, 1971
Arnold AM: Podophyllotoxin derivative VP-16–213. Cancer Chemother Pharmacol 3:71–80, 1979
Issell BF, Crooke ST: Etoposide (VP-16–213): an overview. Cancer Treat Rev 6:107–124, 1979
O'Dwyer PJ, Leyland-Jones B, Alonso MT, Marsoni S, Wittes RE: Etoposide (VP-16–213): Current status of an active anticancer drug. New Eng J Med 313:692–700, 1985
European Organization for Research on the treatment of Cancer, Clinic Screening Group: Epipodophyllotoxin VP-16- 213 in treatment of acute leukemias, hematosarcomas and solid tumors. Brit Med J 3:199–202, 1973
Radice PA, Bunn PA, Ihde DC: Therapeutic trials with VP-16–213 and VM-26: Active agents in small cell lung cancer, non-Hodgkin's lymphomas and other malignancies. Cancer Treat Rep 63:1231–1239, 1979
Nissen NI, Pajak TF, Leone LA, Bloomfield CD, Kennedy BJ, Ellison RR, Silver RT, Weiss RB, Cuttner J, Falkson G, Kung F, Bergevin PR, Holland JF: Clinical trial of VP-16–213 (NSC 141540) IV twice weekly in advanced neoplastic disease. A study by the Cancer and Leukemia Group B. Cancer 45:232–235, 1980
Vogelzang NJ, Raghaven D, Kennedy BJ: VP-16–213 (Etoposide): The mandrake root from Issyk-Kul. Am J Med 71:136–144, 1982
Jungi WF: Etoposide single agent chemotherapy for solid tumors. Cancer Treatment Rev 9:31–37 (Suppl A), 1982
Tirelli U, D'Incalci D, Canetta R, Tumolo S, Franachi G, Veronesi A, Galligioni E, Trovo MG, Rossi C, Grigoletto E: Etoposide (VP-16–213) in Malignant Brain Tumors: A Phase II Study. J of Clin Oncol 2:432–437, 1984
Rivera G, Avery T, Pratt C: 4′-demethylepipodophyllotoxin 9-(4–6–0–2-thenylidene-B-D-gludopyranoside) (NSC-122819; VM-26) and 4′-demethylepipodophyllotoxin 9-(4,6–0-ethylidene-B-D-glucopyranoside) (NSC-141540; VP-16–213) in childhood cancer: Preliminary observations. Cancer Chemother Rep 59:743–749, 1975
Chard Jr RL, Krivit W, Bleyer WA, Hammond D: Phase II study of VP-16–213 in childhood malignant disease: A Children's Cancer Study Group Report. Cancer Treat Rep 63:1755–1759, 1979
Hayes FA, Green A, Thompson E, Wilimas J, Etcubanas E, Pratt C: Phase II Trial of VP-16–213 in Pediatric Solid Tumors. (Abstract) Proc Amer Soc Clin Oncol 2:66 (C-256), 1983
Eagan RT, Carr DT, Frytak S, Rubin J, Lee RE: VP-16–213 versus Polychemotherapy in Patients with Advanced Small Cell Lung Cancer. Cancer Treat Rep 60:494–451, 1976
Antman K, Pomfret E, Karp G, Skarin A, Canellos G: Phase II Trial of Etoposide in Previously Treated Small Cell Carcinoma of the Lung. Cancer Treat Rep 68:1413–1414, 1984
Wittes RE, Marsoni S, Simon R, Leyland-Jones B: The Phase II Trial. Cancer Treat Rep 69:1235–1239, 1985
Abromowitch M, Bowman WP, Ochs J, Rivera G: Etoposide (VP-16) with prednisone and vincristine for the treatment of refractory acute lymphoblastic leukemia. J Clin Oncol 3:789–792, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kung, F., Hayes, F.A., Krischer, J. et al. Clinical trial of etoposide (VP-16) in children with recurrent malignant solid tumors. Invest New Drugs 6, 31–36 (1988). https://doi.org/10.1007/BF00170776
Issue Date:
DOI: https://doi.org/10.1007/BF00170776