Summary
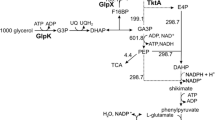
The construction and characterization of glyceraldehyde-3-phosphate-dehydrogenase (GPD) overproducing transformants of Aspergillus nidulans and their behaviour in acetate-limited continuous cultures and glucose-grown batch cultures are described. The A. nidulans acetamidase deletion strain MH1277 was transormed with the homologous gpdA gene on a vector with the homologous acetamidase-gene (amdS) as a selection marker. Transformant Al contains about nine integrated copies of the gpdA gene, and shows a proportional gene-dosage GPD production of about 22% of the total soluble cell protein. Compared to the wild-type MH1277, Al has higher growth yields and reaches higher specific growth rates on both acetate and glucose, which could be due to the key position of GPD in glycolysis and gluconeogenesis.
Similar content being viewed by others
References
Aar PC van der, Lopes TS, Klootwijk J, Groeneveld Ph, Van Verseveld HW, Stouthamer AH (1990) Consequences of phosphoglycerate kinase overproduction for the growth and physiology of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 32:577–587.
Babel WJ, Muller RH (1985) Correlation between cell composition and carbon conversion efficiency in microbial growth: a theoretical study. Appl Microbiol Biotechnol 22:201–207.
Bergmeyer HU (1974) Methods of enzymatic analysis, vol 2, 2nd edn. Academic Press, New York, p 467.
Brammar WJ, Clarke PH (1964) Induction and repression of Pseudomonas aeruginosa amidase. J Gen Microbiol 37:307–319.
Bull AT (1970) Chemical composition of wild type and mutant Aspergillus nidulans cell walls. The nature of polysaccharide and melanin constituents. J Gen Microbiol 63:75–94.
Bulthuis BA (1990) Stoichiometry of growth and product formation in Bacillus licheniformis. Ph.D thesis, Vrije Universiteit, Amsterdam.
Bulthuis BA, Frankena J, Koningstein GM, Van Verseveld HW, Stouthamer AH (1988) Instability of protease production in a rel+/rel— pair of Bacillus licheniformis and associated morphological and physiological characteristics. Antonie van Leeuwenhoek 54:95–111.
Carter BLA, Bull AT (1971) The effect of oxygen tension in the medium on the morphology and growth kinetics of Aspergillus nidulans. J Gen Microbiol 65:265–273.
Carter BLA, Bull AT, Pirt SJ, Rowley BI (1971) Relationship between energy substrate utilization and specific growth rate in Aspergillus nidulans. J Bacteriol 108:309–313.
Fincham JRS (1989) Transformation in fungi. Microbiol Rev 53:148–170.
Finkelstein DB (1987) Improvement of enzyme production in Aspergillus. Antonie van Leeuwenhoek 53:349–352.
Gopal CV, Broad D, Lloyd D (1989) Bioenergetic consequences of protein overexpression in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 30:160–165.
Hynes MJ, Corrick CM, King JA (1983) Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol 3:1430–1439.
Laemmli UK, Farve M (1973) Maturation of the head of bacteriophage T4. I. DNA packing events. J Mol Biol 80:575–599.
Lipmann F, Tuttle LC (1945) A specific micromethod for the determination of acylphosphates. J Biol Chem 159:21–28.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275.
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
McCullough W, Roberts CF, Osmani SA, Scrutton MC (1986) Regulation of carbon metabolism in filamentous fungi. In: Morgan MJ (ed) Carbohydrate metabolism in cultured cells. Plenum Press, New York, pp 287–355.
Pitt DE, Bull AT (1982) Influence of culture conditions on the physiology and composition of Trichoderma aureoviride. J Gen Microbiol 128:1517–1527.
Postma E, Verduyn C, Scheffers WA, Dijken JP van (1989) Enzymatic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 55:468–477.
Punt PJ, Dingemanse MA, Jacobs-Meijsing BJM, Pouwels PH, Van den Hondel CAMJJ (1988) Isolation and characterization of the glyceraldehyde-3-phosphate dehydrogenase gene of Aspergillus nidulans. Gene 69:49–57.
Roels JA (1981) The application of macroscopic principles to microbial metabolism. Ann NY Acad Sci 369:113–134.
Rowley BI, Pirt SJ (1972) Melanin production by Aspergillus nidulans in batch and chemostat cultures. J Gen Microbiol 72:553–563.
Rowley BI, Bull AT (1973) Chemostat for the cultivation of moulds. Lab Prac 22:286–289.
Saunders G, Picknett TM, Tuite MF, Ward M (1989) Heterologous gene expression in filamentous fungi. Tibtech 7:283–287.
Schellart JA (1975) The utilization of ethanol, lactic acid and acetic acid. Fungal protein from corn waste effluents. Ph.D thesis, Landbouw Universiteit, Wageningen, pp 39–45.
Schreferl-Kunar G, Grotz M, Rohr M, Kubicek CP (1989) Increased citric acid production by mutants of Aspergillus niger with increased glycolytic capacity. FEMS Microbiol Lett 59:297–300.
Stouthamer AH (1973) A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antoine van Leeuwenhoek 39:545–565.
Stouthamer AH (1979) The search for correlation between theoretical and experimental growth yields. Microb Biochem 21:1–47.
Vries W de, Stouthamer AH (1968) Fermentation of glucose, lactose, galactose, mannitol, and xylose by bifidobacteria. J Bacteriol 96:472–478.
Wernars K, Goosen T, Wennekes LMJ, Visser J, Bos CJ, Van den Broek HWJ, Gorcom RFM, Van den Hondel CAMJJ, Pouwels PH (1985) Gene amplification in Aspergillus nidulans by transformation with vectors containing the amdS gene. Curr Genet 9:361–368.
Wernars K, Goosen T, Van den Hondel CAMJJ, Wennekes LMJ, Van den Broek HWJ (1986) The effect of the A. nidulans ans1 sequence on Aspergillus transformation. DNA-mediated transformation of the filamentous fungus Aspergillus nidulans. Ph.D thesis, Landbouw Universiteit, Wageningen, pp 100–116.
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119.
Yelton MM, Hamer JE, Timberlake WE (1984) Transformation of Aspergillus nidulans using a trpC plasmid. Proc Natl Acad Sci USA 81:1470–1474.
Author information
Authors and Affiliations
Additional information
Offprint requests to: P. P. F. Hanegraaf
Rights and permissions
About this article
Cite this article
Hanegraaf, P.P.F., Punt, P.J., van den Hondel, C.A.M.J.J. et al. Construction and physiological characterization of glyceraldehyde-3-phosphate dehydrogenase overproducing transformants of Aspergillus nidulans . Appl Microbiol Biotechnol 34, 765–771 (1991). https://doi.org/10.1007/BF00169347
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169347