Summary
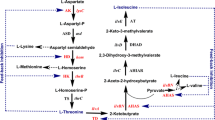
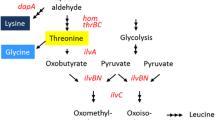
The hom-thrB operon (homoserine dehydrogenase/homoserine kinase) and the thrC gene (threonine synthase) of Corynebacterium glutamicum ATCC 13 032 and the hom FBR (homoserine dehydrogenase resistant to feedback inhibition by threonine) alone as well as hom FBR-thrB operon of C. glutamicum DM 368-3 were cloned separately and in combination in the Escherichia coli/C. glutamicum shuttle vector pEK0 and introduced into different corynebacterial strains. All recombinant strains showed 8- to 20-fold higher specific activities of homoserine dehydrogenase, homoserine kinase, and/or threonine synthase compared to the respective host. In wild-type C. glutamicum, amplification of the threonine genes did not result in secretion of threonine. In the lysine producer C. glutamicum DG 52-5 and in the lysine-plus-threonine producer C. glutamicum DM 368-3 overexpression of hom-thrB resulted in a notable shift of carbon flux from lysine to threonine whereas cloning of hom FBR-thrB as well as of hom FBR in C. glutamicum DM 368-3 led to a complete shift towards threonine or towards threonine and its precursor homoserine, respectively. Overexpression of thrC alone or in combination with that of hom FBR and thrB had no effect on threonine or lysine formation in all recombinant strains tested.
Similar content being viewed by others
References
Bell SC, Turner JM (1976) Bacterial catabolism of threonine; threonine degradation initiated by l-threonine-NAD+ oxidoreductase. Biochem J 156:449–458
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cohen SN, Chang ACY, Hsu L (1973) Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-plasmid DNA. Proc Natl Acad Sci USA 69:2110–2114
Cremer J, Treptow C, Eggeling L, Sham H (1988) Regulation of enzymes of lysine biosynthesis in Corynebacterium glutamicum. J Gen Microbiol 134:3221–3229
Follettie MT, Sinskey AJ (1986) Molecular cloning and nucleotide sequence of the Corynebacterium glutamicum pheA gene. J Bacteriol 167:695–702
Follettie MT, Shin HK, Sinskey AJ (1988) Organization and regulation of the Corynebacterium glutamicum hom-thrB and thrC loci. Mol Microbiol 2:53–62
Han KS, Archer JAC, Sinskey AJ (1990) The molecular structure of the Corynebacterium glutamicum threonine synthase gene. Mol Microbiol 4:1693–1702
Ishida M, Yoshino E, Makihara R, Sato K, Enei H, Nakamori S (1989) Improvement of an l-threonine producer derived from Brevibacterium flavum using threonine operon of Escherichia coli K-12. Agric Biol Chem 53:2269–2271
Katsumata R, Mizukami T, Kikuchi Y, Kino K (1986) Threonine production by the lysine producing strain of Corynebacterium glutamicum with amplified threonine biosynthetic operon. In: Alacevic M, Hranueli D, Toman Z (eds) Genetics of industrial microorganisms B. Pliva, Zagreb, pp 217–226
Kinoshita S (1985) Glutamic acid bacteria. In: Demain AL, Solomon NA (eds) Biology of industrial microorganisms. Benjammin/Cummings Publishing Company, London, pp 115–142
Kleemann A, Leuchtenberger W, Hoppe B, Tanner H (1985) Amino acids. In: Bartholomé E, Biekert E, Hellmann H (eds) Ullmann's encyclopedia of industrial chemistry A2. VCH-Verlagsgesellschaft, Weinheim, pp 57–97
Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 63:607–613
Lennox ES (1955) Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206
Liebl W, Bayerl A, Schein B, Stillner U, Schleifer KH (1989) High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol Lett 65:299–304
Martin JF, Santamaria R, Sandoval H, Real G del, Mateos LM, Gil JA, Aguilar A (1987) Cloning system in amino acid producing corynebacteria. Bio Technology 5:137–146
Mateos LM, Real G del, Aguilar A, Martin JF (1987a) Cloning and expression in Escherichia coli of the homoserine kinase (thrB) gene from Brevibacterium lactofermentum. Mol Gen Genet 206:361–367
Mateos LM, Real G del, Aguilar A, Martin JF (1987b) Nucleotide sequence of the homoserine dehydrogenase (thrA) gene of Brevibacterium lactofermentum. Nucleic Acids Res 15:10598
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y.
Miyajima R, Shiio I (1970) Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum; III. Properties of homoserine dehydrogenase. J Biochem 68:311–319
Miyajima R, Shiio I (1971) Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum; IV. Repression of the enzymes in threonine biosynthesis. Agric Biol Chem 35:424–430
Miyajima R, Otsuka SI, Shiio I (1968) Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum; I. Inhibition by amino acids of the enzymes in threonine biosynthesis. J Biochem 63:139–148
Morinaga Y, Takagi H, Ishida M, Miwa K, Sato T, Nakamori S, Sano K (1987) Threonine production by coexistence of cloned genes coding homoserine dehydrogenase and homoserine kinase in Brevibacterium lactofermentum. Agric Biol Chem 51:93–100
Peoples OP, Liebl W, Bodis M, Maeng PJ, Follettie MT, Archer JA, Sinskey AJ (1988) Nucleotide sequence and fine structural analysis of the Corynebacterium glutamicum hom-thrB operon. Mol Microbiol 2:53–62
Sano K, Shiio I (1970) Microbial production of l-lysine. III. Production by mutants resistant to S-(2-aminoethyl)-l-cysteine. J Gen Appl Microbiol 16:373–391
Shames SL, Wedler FC (1984) Homoserine kinase of Escherichia coli: kinetic mechanism and inhibition by l-aspartate semialdehyde. Arch Biochem Biophys 235:359–370
Shiio I, Miyajima R (1969) Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem 65:849–859
Shiio I, Miyajima R, Nakamori S (1970) Homoserine dehydrogenase genetically desensitized to the feedback inhibition in Brevibacterium flavum. J Biochem 68:859–866
Skarstedt MT, Greer SB (1973) Threonine synthetase of Bacillus subtilis. J Biol Chem 248:1032–1044
Theze J, Saint-Girons I (1974) Threonine locus of Escherichia coli K-12. Genetic structure and evidence for an operon. J Bacteriol 118:990–998
Tosaka O, Ishihara M, Morinaga Y, Takinami K (1979) Mode of conversion of asparto β-semialdehyde to l-threonine and l-lysine in Brevibacterium lactofermentum. Agric Biol Chem 43:265–270
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Author information
Authors and Affiliations
Additional information
Offprint requests to: B. J. Eikmanns
Rights and permissions
About this article
Cite this article
Eikmanns, B.J., Metzger, M., Reinscheid, D. et al. Amplification of three threonine biosynthesis genes inCorynebacterium glutamicum and its influence on carbon flux in different strains. Appl Microbiol Biotechnol 34, 617–622 (1991). https://doi.org/10.1007/BF00167910
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00167910