Abstract
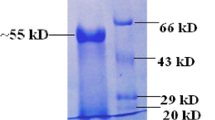
Trichoderma reesei was studied for its ability to produce β-mannanase activity on a variety of carbon sources. The highest β-mannanase activity was produced on cellulose, whereas β-mannan-containing carbon sources (such as kojac powder or locust bean gum) gave lower enzyme titres. The enzyme responsible for the major β-mannanolytic activity from T. reesei was purified to physical homogeneity by preparative chromatofocusing and anion exchange fast protein liquid chromatography. This β-mannanase is a glycoprotein, with a molecular mass of 46 (±2) kDa and an isoelectric point of 5.2. It has an optimal pH at 5.0 and broad pH stability (2.5–7.0). It is stable for 60 min at 55° C, and has an optimal temperature for activity at 75° C. During incubation with locust bean gum, the enzyme releases mainly tri- and disaccharides.
Similar content being viewed by others
References
Akino T, Kato C, Horikoshi K (1989) The cloned β-mannanase gene from alkalophilic Bacillus sp. AM-001 produces two β-mannanase in Escherichia coli. Arch Microbiol 152:10–15
Araujo A, Ward OP (1991) Studies on the galactomannan-degrading enzymes produced by Sporotrichum cellulophilum. J Ind Microbiol 8:229–236
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buchert J, Siika-Aho M, Kantelinen A, Ranua M, Viikari L (1992) Synergism of oylanases and mannanases in the treatment of pulp. In: Kuwahara M, Shimada M (eds) Abstracts of the Fifth International Conference on Biotechnology in the Pulp and Paper Industry, Kyoto, May 1992. P 18
Civas A, Eberhard, Le Dizet P, Petek F (1984) Glycosidases induced in Aspergillus tamarii: secreted α-d-galactosidase and β-d-mannanase. Biochem J 219:857–863
Clark TA, McDonald AG, Senior DJ, Mayers PR (1990) Mannanase and xylanase treatment of softwood chemical pulps: effect on pulp properties and bleachability. In: Kirk TK, Chang HM (eds) Biotechnology in the pulp and paper industry. Butterworth-Heineman, Boston, pp 137–144
Decker RFH, Richards GN (1976) Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem 32:277–352
Eriksson KEL, Winell M (1968) Purification and characterization of a fungal β-mannanase. Acta Chem Scand 22:1924–1934
Eriksson KEL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer Berlin Heidelberg New York
Ishihara M, Shimizu K (1980) Hemicellulases of the brown rotting fungus Tyromyces palustris. IV. purification and some properties of an extracellular mannanase. Mokuzai Gakkaishi 26:811–818
Kubicek CP (1992) The cellulase proteins of Trichoderma reesei: structure, multiplicity, mode of action and regulation of formation. Adv Biochem Eng Biotechnol 45:1–28
Kusakabe I, Park GG, Kumita N, Yasui T, Murakami K (1988) Specificity of β-mannanase from Penicillium purpurogenum for konjac glucomannan. Agric Biol Chem 52:519–524
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lüthi E, Jasmat NB, Grayling RA, Love DR, Bergquist PL (1991) Cloning, sequence analysis, and expression in Escherichia coli of a gene coding for a β-mannanase from the extremely thermophilic bacterium Caldocellum saccharolyticum. Appl Environ Microbiol 57:694–700
Lyr H (1963) Über das Vorkommen von Mannanase bei Pilzen. Z Allg Mikrobiol 3:25–36
McCleary BV (1988) β-d-Mannanases. Methods Enzymol 160:596–610
Park GG, Kusakabe I, Komatsu Y, Kobyashi H, Yasui T, Murakami K (1987) Purification and properties of β-mannanase from Penicillium purpurogenum. Agric Biol Chem 51:2709–2716
Pavlova IN, Tinyanova NZ (1980) Isolation and characterization of microorganisms with mannanase activity. Prikl Biokhim Mikrobiol 16:578–583
Poutanen K, Rättö M, Puls J, Viikari L (1987) Evaluation of different microbial xylanolytic systems. J Biotechnol 6:49–60
Rättö M, Poutanen K (1988) Production of mannan-degrading enzymes. Biotechnol Lett 10:661–664
Reese ET, Shibata Y (1965) β-Mannanases of fungi. Can J Microbiol 11:167–183
Sjöström E (1981) Wood chemistry: fundamentals and applications. Academic Press, New York
Torrie JP, Senior DJ, Saddler JN (1990) Production of β-mannanases by Trichoderma harzianum E58. Appl Microbiol Biotechnol 34:303–307
Wong KKY, Saddler JN (1992) Trichoderma xylanases, their properties and applications. In: Visser J, Beldman G, Kusters-van Someren MA, Voragen AGJ (eds) Xylan and xylanases. Elsevier, Amsterdam, pp 171–186
Author information
Authors and Affiliations
Additional information
Correspondence to: C. P. Kubicek
Rights and permissions
About this article
Cite this article
Arisan-Atac, I., Hodits, R., Kristufek, D. et al. Purification, and characterization of a β-mannanase of Trichoderma reesei C-30. Appl Microbiol Biotechnol 39, 58–62 (1993). https://doi.org/10.1007/BF00166849
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166849