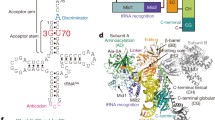
Abstract
Previous sequence analyses have suggested the existence of two distinct classes of aminoacyl-tRNA synthetase. The partition was established on the basis of exclusive sets of sequence motifs (Eriani et al. [1990] Nature 347:203–306). X-ray studies have now well defined the structural basis of the two classes: the class I enzymes share with dehydrogenases and kinases the classic nucleotide binding fold called the Rossmann fold, whereas the class II enzymes possess a different fold, not found elsewhere, built around a six-stranded antiparallel β-sheet. The two classes of synthetases catalyze the same global reaction that is the attachment of an amino acid to the tRNA, but differ as to where on the terminal adenosine of the tRNA the amino acid is placed: class I enzymes act on the 2′ hydroxyl whereas the class II enzymes prefer the 3′ hydroxyl group. The three-dimensional structure of aspartyl-tRNA synthetase from yeast, a typical class II enzyme, is described here, in relation to its function. The crucial role of the sequence motifs in substrate binding and enzyme structure is high-lighted. Overall these results underline the existence of an intimate evolutionary link between the aminoacyl-tRNA synthetases, despite their actual structural diversity.
Similar content being viewed by others
References
Anselme J, Härtlein M (1989) Asparaginyl-tRNA synthetase from Escherichia coli has significant sequence homologies with yeast aspartyl-tRNA synthetase. Gene 84:481–485
Akins RA, Lambowitz AM (1987) A protein required for splicing group I introns in Neurospora crassa mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell 50:331–345
Artymiuk PJ, Rice DW, Poirrette AR, Willet P (1994) A tale of two synthetases. Nature Structural Biology 1:758–760
Biou V, Yaremchuk A, Tukalo M, Cusack S (1994) The 2.9 Å crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNASer. Science 263:1404–1410
Cassio D, Waller JP (1971) Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem 20:283–300
Cavarelli J, Eriani G, Rees B, Ruff M, Boeglin M, Mitschler A, Martin F, Gangloff J, Thierry JC, Moras D (1994) The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J 13:327–337
Cavarelli J, Rees B, Ruff M, Thierry JC, Moras D (1993) Yeast tRNAAsp recognition by its cognate class II aminoacyl-tRNA synthetase. Nature 362:181–184
Carter G (1993) Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu Rev Biochem 62:715–748
Cerini C, Kerjan P, Astier M, Gratecos D, Mirande M, Semeria MA (1991) Component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J 10:4267–4277
Cusack S, Berthet-Colominas C, Härtlein M, Nassar N, Leberman R (1990) A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature 347:249–255
Cusack S, Härtlein M, Leberman R (1991) Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res 19:3489–3498
Eriani G, Delarue M, Poch O, Gangloff J, Moras D (1990) Partition of synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347:203–206
Eriani G, Dirheimer G, Gangloff J (1991) Cysteinyl-tRNA synthetase: determination of the last E. coli aminoacyl-tRNA synthetase primary structure. Nucleic Acids Res 19:265–269
Eriani G, Cavarelli J, Martin F, Dirheimer G, Moras D, Gangloff J (1993) Role of dimerization in yeast aspartyl-tRNA synthetase and importance of the class II invariant proline. Proc Natl Acad Sci USA 90:10816–10820
Fraser FT, Rich A (1975) Amino acids are not all initially attached to the same position on transfer RNA molecules. Proc Natl Acad Sci USA 72:3044–3048
Freist W, Sternbach H, Cramer F (1987) Isoleucyl-tRNA synthetase from baker's yeast and from Escherichia coli MRE600. Eur J Biochem 169:33–39
Freist W, Sternbach H (1988) Tyrosyl-tRNA synthetase from baker's yeast. Order of substrate addition, discrimination of 20 amino acids in aminoacylation of tRNA TyrCCA and tRNA TyrCCA(3′NH2). Eur J Biochem 177:425–433
Freist W, Sternbach H, Cramer F (1989) Arginyl-tRNA synthetase from yeast. Discrimination between 20 amino acids in aminoacylation of tRNA ArgCCA and tRNA ArgCCA(3′NH2). Eur J Biochem 186:535–541
Gatti PL, Tzagoloff A (1991) Structure and evolution of a group of related aminoacyl-tRNA synthetases. J Mol Biol 218:557–568
Griffin BE, Jarmen M, Reese CB, Sulston JE, Trentham DR (1966) Some observations relating to acyl mobility in aminoacyl soluble ribonucleic acids. Biochemistry 5:3638–3649
Guildo MD (1993) Origin glutaminyl-tRNA synthetase: an example of palimpset? J Mol Evol 37:5–10
Hountondji C, Dessen P, Blanquet S (1986) Sequence similarities among the family of aminoacyl-tRNA synthetases. Biochimie 68: 1071–1078
Igloi GL, von der Haar F, Cramer F (1978) Aminoacyl-tRNA synthetases from yeast: generality of chemical proofreading in the prevention of misaminoacylation of tRNA. Biochemistry 17:3459–3467
Kraulis PJ (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr 24: 946–950
Lévêque F, Plateau P, Dessen P, Blanquet S (1990) Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res 18:305–312
Lu Y, Hill KAW (1994) The invariant arginine of motif 2 of Escherichia coli alanyl-tRNA synthetase is important for catalysis but not for substrate binding. J Biol Chem 269:12137–12141
Mosyak L, Saffro M (1993) Phenylalanyl-tRNA synthetase from Thermus thermophilus has four antiparallel folds of which only two are catalytically functional. Biochimie 75:1091–1098
Ribas de Pouplana L, Buechter DD, Davis MW, Schimmel P (1993) Idiographic representation of conserved domain of a class II tRNA synthetase of unknown structure. Protein Sci 2:2259–2262
Risler JL, Zelver C, Brunie S (1981) Methionyl-tRNA synthetase shows the nucleotide binding fold observed in dehydrogenases. Nature 292:385–386
Rossmann MG, Moras D, Osen KW (1974) Chemical and biological evolution of a nucleotide binding domain. Nature 250:194–199
Rould MA, Perona JJ, Söll D, Steitz TA (1989) Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNAGln and ATP at 2,8 Å resolution. Science 246:1135–1142
Rubin J, Blow D (1981) Amino acid activation in crystalline tyrosyl-tRNA synthetase from Bacillus stearothermophilus. J Mol Biol 145:489–500
Ruff M, Krishnaswamy S, Boeglin M, Poterszman A, Mitschler A, Rees B, Thierry JC, Moras D (1991) Class II aminoacyl-transfer RNA synthetase: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNAAsp. Science 252:1682–1689
Schimmel P, Söll D (1979) Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Ann Rev. Biochem 48: 601–648
Sellami M, Chatton B, Fasiolo F, Dirheimer G, Ebel JP, Gangloff J (1986) Nucleotide sequence of the gene coding for yeast cytoplasmic aspartyl-tRNA synthetase (APS); mapping of the 5′ and 3′ termini of AspRS mRNA. Nucleic Acids Res 14:1657–1666
Sonnebom TM (1965) In: Bryson V, Vogel HJ (eds) Evolving genes and proteins. Academic Press, New York, pp 337–397
Sprinzl M, Cramer F (1975) Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2′- or 3′-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci USA 72:3049–3053
von der Haar F, Cramer F (1976) Hydrolytic action of aminoacyl-tRNA synthetases from baker's yeast: “chemical proofreading” preventing acylation of tRNAIle with misactivated valine. Biochemistry 15:4131–4138
Webster TA, Tsai H, Kula M, Mackie GA, Schimmel P (1984) Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science 226:1315–1317
Woese C (1965) The genetic code. Harper and Row, New York, pp 156–160
Wong JTF (1975) A co-evolution theory of the genetic code. Proc Natl Acad Sci USA 72:1909–1912
Author information
Authors and Affiliations
Additional information
Based on a presentation made at a workshop— “Aminoacyl-tRNA Synthetases and the Evolution of the Genetic Code”—held at Berkeley, CA, July 17–20, 1994
Correspondence to: G. Eriani
Rights and permissions
About this article
Cite this article
Eriani, G., Cavarelli, J., Martin, F. et al. The class II aminoacyl-tRNA synthetases and their active site: Evolutionary conservation of an ATP binding site. J Mol Evol 40, 499–508 (1995). https://doi.org/10.1007/BF00166618
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00166618