Summary
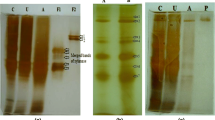
Two xylanolytic enzymes, xylanase and β-xylosidase from the yeast Pichia stipitis were purified to homogeneity and characterized. Both enzymes are secreted into the culture medium upon growth on xylan. The xylanase is a glycoprotein with an approximate molecular mass of 43 kDa. The N-linked carbohydrate content was estimated to be 26% by endoglycosidase H digestion. The β-xylosidase protein has a molecular mass of 37 kDa as determined by sodium dodecyl sulphate gel electrophoresis. Synthesis of xylanase was found to be inducible by xylan and repressible by xylose and glucose. By contrast, β-xylosidase is synthesized constitutively to a considerable degree. The purified β-xylosidase is able to hydrolyse aryl-β-D-glucosides with an even higher rate than β-xylosides. Thus, this enzyme may not be a specific component of the xylan-degrading system of P. stipitis.
Similar content being viewed by others
References
Ballou CE (1990) Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol 185:440–470
Berenger JF, Frixon C, Bigliardi J, Creuzet N (1985) Production, purification, and properties of thermostable xylanase from Clostridium stercorarium. Can J Microbiol 31:635–643
Biely P (1985) Microbial xylanolytic systems. Trends Biotechnol 3:286–290
Biely P, Vrsanská M, Krátky Z (1980a) Xylan-degrading enzymes of the yeast Cryptococcus albidus. Eur J Biochem 108:313–321
Biely P, Krátky Z, Vrsanská M, Urmanicová D (1980b) Induction and inducers of endo-1,4-β-xylanase in the yeast Cryptococcus albidus. Eur J Biochem 108:323–329
Biely P, Mislovicova D, Toman R (1985) Soluble chromogenic substrates for the assays of endo-1,4-β-xylanases and endo-1,4-β-glucanases. Anal Biochem 144:142–146
Blake MS, Johnston KH, Russel-Jones GJ, Gottschlich EC (1984) A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem 136:175–179
Bluhm H, Beier M, Gross MJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bradford MM (1976) A rapid sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ciriacy M (1975) Genetics of alcohol dehydrogenase in Saccharomyces cerevisiae. I. Isolation and genetic analysis of adh mutants. Mutat Res 29:315–326
Dekker RFH, Richards GN (1976) Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem 32:277–352
Jeffries TW (1990) Fermentation of D-xylose and cellobiose. In: Verachtert H, De Mot R (eds) Yeast biotechnology and biocatalysis, vol 5. Dekker, New York, p 376
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Leathers TD (1988) Amino acid composition and partial sequence of xylanase from Aureobasidium. Biotechnol Lett 10:775–780
Leathers TD, Kurtzman CP, Detroy RW (1984) Overproduction and regulation of xylanase in Aureobasidium pullulans and Cryptococcus albidus. Biotechnol Bioeng Symp 14:225–240
Leclerc M, Arnaud A, Ratomaheninar, Galzy P (1987) Yeast β-glucosidases. Biotechnol Genet Eng Rev 5:269–295
Lee H, Biely P, Latta RK, Barbosa MFS, Schneider H (1986) Utilization of xylan by yeasts and its conversion to ethanol by Pichia stipitis. Appl Environ Microbiol 52:320–324
McCarthy AJ (1987) Lignocellulose-degrading actinomycetes. FEMS Microbiol Rev 46:145–163
Mishra C, Keskar S, Rao M (1984) Production and properties of extracellular xylanase from Neurospora crassa. Appl Environ Microbiol 48:224–228
Morosoli R (1985) Molecular expression of xylanase gene in Cryptococcus albidus. Biochim Biophys Acta 826:202–207
Morosoli R, Roy C, Yaguchi M (1986) Isolation and partial primary sequence of a xylanase from the yeast Cryptococcus albidus. Biochim Biophys Acta 870:473–478
Morosoli R, Durand S, Boucher F (1989) Stimulation of xylanase synthesis in Cryptococcus albidus by cyclic AMP. FEMS Microbiol Lett 57:57–60
Poutananen K, Puls J (1988) Characteristics of Trichoderma reesei β-xylosidase and its use in the hydrolysis of solubilized xylans. Appl Microbiol Biotechnol 28:425–432
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Uziie M, Matsuo M, Yasui T (1985) Purification and some properties of Chaetomium trilaterale β-xylosidase. Agric Biol Chem 49:1159–1166
Whistler RL, Richards EL (1970) Hemicelluloses. In: Pigman W, Horton D (eds) The carbohydrates — chemistry and biochemistry, vol 2A, 2nd edn. Academic Press, New York, pp 447–469
Wilkonson JM (1987) Dansyl amino acids. In: Hancock WS (ed) CRC Handbook for HPLC for separation of amino acids, peptides and proteins, vol 1. CRC Press, Boca Raton, Florida, pp 339–350
Woodward J (1984) Xylanases: functions, properties and applications. Top Enzyme Ferment Biotechnol 8:9–30
Wong KKY, Tan LUL, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Author information
Authors and Affiliations
Additional information
Offprint requests to: M. Ciriary
Rights and permissions
About this article
Cite this article
Özcan, S., Kötter, P. & Ciciary, M. Xylan-hydrolysing enzymes of the yeast Pichia stipitis . Appl Microbiol Biotechnol 36, 190–195 (1991). https://doi.org/10.1007/BF00164418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164418