Abstract
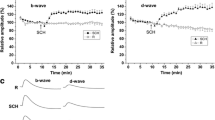
α-aminoadipic acid was intravitreally applied to adult rabbits. After 5 h, the retinae of these animals were examined by electroretinography and histochemistry. The retinal Müller cells were extremely swollen, and the electroretinographic slow P III was extinguished. The mass receptor potential was somewhat diminished. The results are consistent with the opinion that the slow P III is the reaction of the Müller cells to the changed external potassium ion concentration caused by the activity of the photoreceptors.
Similar content being viewed by others
References
Bonaventure N et al (1980) see Welinder, Textorius and Nilsson (1982)
Eränkö O, Niemi M and Merenmies E (1961) Histochemical observations on esterases and oxidative enzymes of the retina. In: Smelser GK (ed): The Structure of the Eye. New York, Academic Press, pp 159–171
Faber D (1969) Analysis of the slow transretinal potentials in response to light. PhD Dissertation, State University, New York
Fujimoto M and Tomita T (1979) Reconstruction of the slow P III from the rod potential. Invest Ophthal Vis Sci 18:1090–1093
Furakawa T and Hanawa I (1955) Effects of some common cations on electroretinogram of the toad. Jap J Physiol 5:289–300
Hanitzsch R (1973) Intraretinal isolation of P III subcomponents in the isolated rabbit retina after treatment with sodium aspartate. Vision Res 13:2093–2102
Hanitzsch R and Bornschein H (1965) Spezielle Überlebensbedingungen für isolierte Netzhäute verschiedener Warmblüter. Experientia 21:1–3
Karwoski CJ and Proenza LM (1977) Relationship between Müller cell responses, a local transretinal potential, and potassium flux. J Neurophysiol 40:244–259
Lund Karlsen R (1978) The toxic effect of sodium glutamate and DL-α-aminoadipic acid on rat retina: changes in high affinity uptake of putative transmitters. J Neurochem 31:1055–1061
Miller RF and Dowling JE (1970) Intracellular responses of the Müller (glial) cells of mudpuppy retina: Their relation to the b-wave of the electroretinogram. J Neurophysiol 33:323–341
Newman EA (1979) B-wave currents in the frog retina. Vision Res 19:227–234
Newman EA (1980) Current source-density analysis of the b-wave of frog retina. J Neurophysiol 43:1355–1366
Oakley B II and Green DG (1976) Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol 39:1117–1133
Oakley B II, Flaming DG and Brown KT (1979) Effect of the rod receptor potential upon retinal extracellular potassium ion concentration. ARVO Abstracts, Invest Ophthal Vis Sci 8 (suppl):4
Olney JW, Ho OL and Rhee V (1971) Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous systems. Exp Brain Res 14:61–76
Pedersen O and Lund Karlsen R (1979) Destruction of Müller cells in the adult rat by intravitreal injection of D, L-α-aminoadipic acid. An electron microscopic study. Exp Eye Res 28:569–575
Reichenbach A and Hanitzsch R (1975) Der Nachweis von Subkomponenten von pIII im ERG der isolierten Kaninchennetzhaut unter Verwendung von Natriumasparaginat. Acta Biol Med Germ 34:857–863
Reichenbach A and Wohlrab F (1983) Quantitative properties of Müller cells in rabbit retina as revealed by histochemical demonstration of NADH-diaphorase activity. Graefe's Arch Clin Exp Ophthal 220:81–83
Sillman AJ, Ito H and Tomita T (1969) Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res 9:1435–1442
Sjöstrand FS and Nilsson SE (1964) The structure of the rabbit retina as revealed by electron microscopy. In: Prince JH (ed): The Rabbit in Eye Research. Springfield, Ill., Charles C.Thomas
Steinberg RH, Oakley B II and Niemeyer G (1979) The light-evoked decrease in (K+)0 in the distal retina of the cat. ARVO Abstracts, Invest Ophthal Vis Sci 18 (suppl):4
Szamier RB, Ripps H and Chapell RL (1980) On the glial-cell origin of the ERG b-wave. ARVO Abstracts, Invest Ophthal Vis Sci. 19 (Suppl): 39
Tomita T (1976) Electrophysiological studies of retinal cell function. Invest Ophthal 15:171–187
Wachtmeister L (1981) Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG). II. Glutamate - aspartate- and dopamin antagonists. Acta Ophthal 59:247–258
Welinder E, Textorius O and Nilsson SEG (1982) Effects of intravitreally injected DL-α-aminoadipic acid on the c-wave of the D.C.-recorded electroretinogram in albino rabbits. Invest Ophthal Vis Sci 23:240–245
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reichenbach, A., Wohlrab, F. Effects of α -aminoadipic acid on the glutamate-isolated P III of the rabbit electroretinogram. Doc Ophthalmol 59, 359–364 (1985). https://doi.org/10.1007/BF00159170
Issue Date:
DOI: https://doi.org/10.1007/BF00159170