Summary
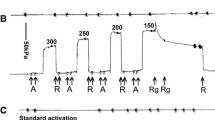
The effects of MgADP and inorganic phosphate (Pi) on cross-bridge detachment were determined in tonic (rabbit femoral artery) and phasic (rabbit bladder and guinea pig portal vein) smooth muscles permeabilized with staphylococcal α-toxin. Relaxation from rigor was induced by photolysis of ATP (1.2–1.5 mm) from caged ATP. The initial one second of relaxation from rigor was resolved into two exponential components: a rapid component with normalized amplitudes, A f, of 8, 15 and 26% and rate constants, kf (in s-1) of 26, 36 and 30 in rabbit femoral artery, guinea pig portal vein, and rabbit bladder; the respective rate constants of the second, slower component, ks, were 0.07, 0.2 and 0.1 Removal of residual endogenous ADP with apyrase treatment increased the amplitude A f and accelerated ks; addition of MgADP reduced A f. The combination of these effects (increases in A f and ks) decreased the t 1/2 of relaxation from control values by factors of 2.6 (femoral artery), 6.7 (portal vein) and 10 (bladder). Pi (30 mm) further increased the amplitudes A f.
The affinity of MgADP for myosin cross-bridges, estimated as the reduction of the relative amplitude of the rapid component, A f, was significantly higher in tonic than in phasic smooth muscle: the K d of MgADP was 1.1±0.3 μm in rabbit femoral artery and 4.9±1.0 μm in rabbit bladder. The higher affinity of tonic smooth muscle myosin for MgADP correlated with its relatively high LC17b isoform content (58±4.2%) in contrast to the lower affinity of the phasic, bladder detrusor smooth muscle that contained only the LC17a isoform. The t 1/2 of relaxation from rigor was markedly slowed down by MgADP (10–200 μm) in femoral artery, but not in bladder smooth muscle. We suggest that:
-
1.
The high affinity of myosin for MgADP and the consequent maintenance of a strongly bound, AM.ADP state by dephosphorylated cross-bridges, as well as cooperative reattachment of unphosphorylated cross-bridges, contribute to the maintenance of ‘latch‘. The acceleration of relaxation by Pi is consistent with the reversal of step(s), including cooperative attachment of nonphosphorylated cross-bridges, that precede entry into strongly bound, force-generating states. The higher affinity for myosin and the greater slowing of relaxation by MgADP in tonic, than phasic, smooth muscle suggest that MgADP may have a greater role in force maintenance at low levels of MLC20 phosphorylation in the tonic muscles.
-
2.
The absence or low expression of the LC17b isoform and/or the presence of a seven amino acid insert near the catalytic site of the myosin heavy chain in phasic smooth muscle may be responsible for their lower affinity for MgADP and for their resultant faster kinetics.
-
3.
The marked slowing by MgADP of the late phases of relaxation from rigor in femoral artery suggests that in tonic smooth muscle the nucleotide affects not only rigor bridges, but also additional state(s) of cooperatively cycling cross-bridges. The lack of effect of MgADP on the later phases of relaxation from rigor in bladder smooth muscle suggests that in this, phasic, smooth muscle MgADP affects primarily cross-bridges in the rigor (AM) state.
Similar content being viewed by others
References
ARNER, A., GOODY, R. S., RAPP, G. & RÜEGG, J. C. (1987) Relaxation of chemically skinned guinea pig taenia coli smooth muscle from rigor by photolytic release of adenosine-5′-triphosphate. J. Muscle Res. Cell Motil. 8, 377–85.
BABIJ, P. (1993) Tissue-specific and developmentally regulated alternative splicing of a visceral isoform of smooth muscle myosin heavy chain. Nucleic Acids Res. 21, 1467–71.
BUTLER, T. M., PACIFICO, D. S. & SIEGMAN, M. J. (1989) ADP release from myosin in permeabilized smooth muscle. Am. J. Physiol. 256, C59–66.
BUTLER, T. M., SIEGMAN, M. J. & MOOERS, S. U. (1983) Chemical energy usage during shortening and work production in mammalian smooth muscle. Am. J. Physiol. 244, C234–42.
BUTLER, T. M., SIEGMAN, M. J., MOOERS, S. U. & NARAYAN, S. R. (1990) Myosin-product complex in the resting state and during relaxation of smooth muscle. Am. J. Physiol. 258, C1092–9.
DANTZIC, J. A., HIBBERD, M. G., TRENTHAM, D. R. & GOLDMAN, Y. E. (1991) Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J. Physiol. 432, 639–680.
DANTZIC, J. A., GOLDMAN, Y. E., MILLAR, N. C., LACKTIS, J. & HOMSHER, E. (1992) Reversal of the cross-bridge forcegenerating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 451, 247–78.
DILLON, P. F., AKSOY, M. O., DRISKA, S. P. & MURPHY, R. A. (1981) Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211, 495–7.
DRISKA, S. P., STEIN, P. G. & PORTER, R. (1989) Myosin dephosphorylation during rapid relaxation of hog carotid artery smooth muscle. Am. J. Physiol. 256, C315–21.
FUGLSANG A., NISHIYE E., SOMLYO A. P. & SOMLYO A. V. (1993) Cooperative reattachment of unphosphorylated cross-bridges may contribute to more extensive ‘latch’ in tonic vs phasic smooth muscle. Biophys. J. 64, A257.
GAGELMANN, M. & GÜTH, K. (1987) Effect of inorganic phosphate on the Ca2+ sensitivity in skinned Taenia coli smooth muscle fibers: Comparison of tension, ATPase activity, and phosphorylation of the regulatory myosin light chains. J. Biophys. 51, 457–63.
GEEVES, M. A. (1991) The dynamics of actin and myosin association and the cross-bridge model of muscle contraction. Biochem. J. 274, 1–14.
GOLDMAN, Y. E., HIBBERD, M. G. & TRENTHAM, D. R. (1984) Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5′-triphosphate. J. Physiol. 354, 577–604.
GONG, M. C., COHEN, P., KITAZAWA, T., IKEBE, M., MASUO, M., SOMLYO, A. P. & SOMLYO, A. V. (1992) Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J. Biol. Chem. 267, 14662–8.
GÜTH, K. & JUNGE, J. (1982) How Ca2+ impedes cross-bridge detachment in chemically skinned Taenia coli. Nature 300, 775–6.
HASEGAWA, Y. & MORITA, F. (1992) Role of 17-kDa essential light chain isoforms of aorta smooth muscle myosin. J. Biochem. 111, 804–9.
HELLSTRAND, P. & ARNER, A. (1985) Myosin light chain phosphorylation and the cross-bridge cycle at low substrate concentration in chemically skinned guinea pig Taenia coli. Pflügers Arch. 405, 323–8.
HELLSTRAND, P. & PAUL, R. J. (1983) Phosphagen content, breakdown during contraction, and O2 consumption in rat portal vein. Am. J. Physiol. 244, C250–8.
HELPER, D. J., LASH, J. A. & HATHAWAY, D. R. (1988) Distribution of isoelectric variants of the 17,000-Dalton myosin light chain in mammalian smooth muscle. J. Biol. Chem. 263, 15748–53.
HIBBERD, M. G. & TRENTHAM, D. R. (1986) Relationships between chemical and mechanical events during muscular contraction. Ann. Rev. Biophys. Biophys. Chem. 15, 119–61.
HIMPENS, B., MATTHJIS, G., SOMLYO, A. V., BUTLER, T. M. & SOMLYO, A. P. (1988) Cytoplasmic free calcium, myosin light chain phosphorylation and force in phasic and tonic smooth muscle. J. Gen. Physiol. 92, 713–29.
HORIUTI, K., SOMLYO, A. V., GOLDMAN, Y. E. & SOMLYO, A. P. (1989) Kinetics of contraction initiated by flash photolysis of caged adenosine triphosphate in tonic and phasic smooth muscles. J. Gen. Physiol. 94, 769–81.
HUXLEY, A. F. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318.
ITOH, T., KANMURA, Y. & KURIYAMA, H. (1986) Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J. Physiol. 376, 231–52.
KAMM, K. E. & STULL, J. T. (1985) Myosin phosphorylation, force, and maximal shortening velocity in neurally stimulated tracheal smooth muscle. Am. J. Physiol. 249, C238–47.
KAWAI, M. & HALVORSON, H. R. (1991) Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys. J. 59, 329–42.
KELLEY, C. A., TAKAHASHI, M., YU, J. H. & ADELSTEIN, R. S. (1993) An insert of seven amino acids confers enzymatic differences between smooth muscle myosins from the intestines and vasculature. J. Biol. Chem. 268, 12848–54.
KERRICK, W. G. & HOAR, PHYLLIS E. (1987) Non-Ca2+-activated contraction in smooth muscle. In Regulation and Contraction of smooth Muscle (edited by SIEGMAN, M. J., SOMLYO, A. P. & STEPHENS, N. L.) pp. 437–48. Alan R. Liss, Inc.
KITAZAWA, T., GAYLINN, B. D., DENNEY, G. H. & SOMLYO, A. P. (1991) G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 266, 1708–15.
KRISANDA, J. M. & PAUL, R. J. (1983) Phosphagen and metabolite content during contraction in porcine carotid artery. Am. J. Physiol. 244, C385–90.
LU, Z., MOSS, R. L. & WALKER, J. W. (1993) Tension transients initiated by photogeneration of MgADP in skinned skeletal muscle fibers. J. Gen. Physiol. 101, 867–88.
MALMQVIST, U. & ARNER, A. (1991) Correlation between isoform composition of the 17 kDa myosin light chain and maximal shortening velocity in smooth muscle. Eur. J. Physiol. 418, 523–30.
MARSTON, S. B. & TAYLOR, E. W. (1980) Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J. Mol. Biol. 139, 573–600.
MILLAR, N. C. & HOMSHER, E. (1990) The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers: a steady-state and transient kinetic study. J. Biol. Chem. 265, 20234–40.
NISHIMURA, J. & VANBREEMEN, C. V. (1991) Energetic aspects of the regulation of Ca++ sensitivity of permeabilized rabbit mesenteric artery: possible involvement of a second Ca2+ regulatory system in smooth muscle contraction. J. Pharmacol. Exp. Therup. 258, 397–402.
NISHIYE, E., SOMLYO, A. V., TÖRÖK, K. & SOMLYO, A. P. (1993) The effects of MgADP on cross-bridge kinetics: a laser flash photolysis study of guinea pig smooth muscle. J. Physiol. 460, 247–71.
PATE, E. & COOKE, R. (1989) Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflügers Arch. 414, 73–81.
REMBOLD, C. M. & MURPHY, R. A. (1993) Models of the mechanism for crossbridge attachment in smooth muscle. J. Muscle Res. Cell Motil. 14, 325–33.
RÜEGG, J. C. (1986) Calcium in Muscle Activation: A Comparative Approach. Springer-Verlag.
SCHNEIDER, M., SPARROW, M. & RÜEGG, J. D. (1981) Inorganic phosphate promotes relaxation of chemically skinned smooth muscle of guinea pig Taenia coli. Experientia 37, 980–2.
SCHOENBERG, M. & EISENBERG, E. (1987) ADP binding to myosin cross-bridges and its effect on the cross-bridge detachment rate constants. J. Gen. Physiol. 89, 905–20.
SELLERS, J. R. (1985) Mechanism of the phosphorylation-dependent regulation of smooth muscle heavy meromyosin. J. Biol. Chem. 260, 15815–19.
SIEMANKOWSKI, R. F., WISEMAN, M. O. & WHITE, H. D. (1985) ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc. Natl. Acad. Sci. USA 82, 658–62.
SLEEP, J. A. & BURTON, K. (1990) The use of apyrase in caged-ATP experiments. Biophys. J. 57, 542a.
SLEEP, J. A. & HUTTON, R. L. (1980) Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment 1. Biochemistry 19, 1276–83.
SOMLYO, A. P. & SOMLYO, A. V. (1968) Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J. Pharmacol. Exp. Therap. 159, 129–45.
SOMLYO, A. V. & SOMLYO, A. P. (1967) Active state and catch-like state in pulmonary artery. J. Gen. Physiol. 50, 168–9.
SOMLYO, A. V., GOLDMAN, Y. E., FUJIMORI, T., BOND, M., TRENTHAM, D. R. & SOMLYO, A. P. (1988) Cross-bridge kinetics: cooperativity and negatively strained crossbridges in vertebrate smooth muscle. A laser flash photolysis study. J. Gen. Physiol. 91, 165–92.
TAKAHASHI, M. & MORITA, F. (1989) Myosin may stay in EADP species during the catch contraction in scallop smooth muscle. J. Biochem. 106, 868–71.
TWAROG, B. M. (1974) Aspects of smooth muscle function in molluscan catch muscle. Physiol. Rev. 56, 829.
VYAS, T. B., MOOERS, S. U., NARAYAN, S. R., WITHERELL, J. C., SIEGMAN, M. J. & BUTLER, T. M. (1992) Cooperative activation of myosin by light chain phosphorylation in permeabilized smooth muscle. Am. J. Physiol. 263, C210–19.
WARSHAW, D. M., DESROISERS, J. M., WORK, S. S. & TRYBUS, K. M. (1991) Effects of MgATP, MgADP, and Pi on actin movement by smooth muscle myosin. J. Biol. Chem. 266, 24339–43.
WHITE, S., MARTIN, A. E. & PERIASAMY, M. (1993) Identification of a novel smooth muscle myosin heavy chain cDNA: isoform diversity in the S1 head region. Am. J. Physiol. 264, C1252–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fuglasang, A., Khromov, A., Török, K. et al. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. J Muscle Res Cell Motil 14, 666–677 (1993). https://doi.org/10.1007/BF00141563
Issue Date:
DOI: https://doi.org/10.1007/BF00141563