Summary
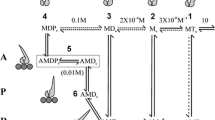
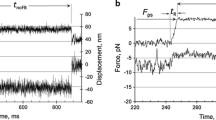
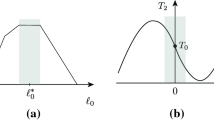
A. F. Huxley's suggestion in Nature (1992) that a structural modification in the myosin head driven by phosphate release can explain the rapid regeneration of the working stroke, which follows the quick recovery elicited by a step release of moderate size (3–6 nm per half-sarcomere), has been tested with a theoretical model. It is assumed that, in the shortening muscle, cross-bridges can undergo their work producing interaction in two ways distinct for the biochemical state and for the amount of filament sliding allowed. During shortening at low speed, as well as after a shortening step of moderate size, phosphate release from the cross-bridge in the AM-ADP-P state promotes a 100 s-1 structural change which resets the myosin head in a configuration that allows for a new complete working stroke in the AM-ADP state. In this case the total sliding distance for interaction is about 15 nm. With the increase in shortening velocity a progressively larger fraction of interacting cross-bridges remains in the AM-ADP-P state throughout the working stroke and the sliding distance for interaction is about 11 nm. Reattachment of detached cross-bridges occurs at moderate rate whichever is the pathway from which they originate. The model predicts satisfactorily the time course of the rapid regeneration of the working stroke in double step experiments, but fails to simulate the transition to the steady state response in staircase experiments, the maximum power output during steady shortening and the decrease in rate of energy liberation at high shortening velocities. These results strengthen the conclusion of our previous modelling work where we demonstrated that the condition necessary to fit the mechanical and energetic properties of shortening muscle is to assume two pathways for cross-bridge cycling distinct for the kinetics of detachment and reattachment.
Similar content being viewed by others
References
BAGSHAWC. R. (1993) Muscle contraction. London: Chapman & Hall.
CHENY. & BRENNERB. (1993) On the regeneration of the actin-myosin power stroke in contracting muscle. Proc. Natl. Sci. USA. 90, 5148–52.
DANTZIGJ. A., GOLDMANY. E., MILLARN. C., LACKTISJ. & HOMSHERE. (1992) Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J. Physiol. 451, 247–78.
EISENBERGE., HILLT. L. & CHEND. Y. (1980) Cross-bridge model of muscle contraction. Quantitative analysis. Bioph. J. 29, 195–227.
FENNW. O. (1924) The relation between work performed and the energy liberated in muscular contraction. J. Physiol. 58, 373–95.
FORDL. E., HUXLEYA. F. & SIMMONSR. M. (1985) Tension transients during steady shortening of frog muscle fibres. J. Physiol. 361, 131–50.
GOLDMANY. E. & SIMMONSR. M. (1977) Active and rigor muscle stiffness. J. Physiol. 269, 55–7P.
HILLA. V. (1938) The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. London Ser. B 126, 136–95.
HILLA. V. (1939) The mechanical efficiency of frogs' muscle. Proc. R. Soc. London Ser. B 127, 434–51.
HILLA. V. (1964a) The effect of load on the heat of shortening of muscle. Proc. R. Soc. London Ser. B 159, 297–318.
HILLA. V. (1964b) The efficiency of mechanical power development during muscular shortening and its relation to load. Proc. R. Soc. London Ser. B 159, 319–24.
HOMSHERE., IRVINGM. & WALLNERA. (1981) Highenergy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. J. Physiol. 321, 423–36.
HUXLEYA. F. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318.
HUXLEYA. F. (1992) A fine time for contractual alterations. Nature 357, 110.
HUXLEYA. F. (1993) In Mechanism of myofilament sliding in muscle contraction (edited by SUGIH. & POLLACKG. H.) pp. 839–47. New York: Plenum Press.
HUXLEYA. F. & SIMMONSR. M. (1971) Proposed mechanism of force generation in striated muscle. Nature 233, 533–8.
HUXLEYH. E. (1969) The mechanism of muscular contraction. Science. 164, 1356–66.
HUXLEYH. E., FARUQIA. R., BORDASJ., KOCHM. H. J. & MILCHJ. R. (1980) The use of synchotron radiation in time-resolved X-ray diffraction studies of myosin layer-line reflections during muscle contraction. Nature 284, 140–3.
IRVINGM. (1995) Give in the filaments. Nature 374, 14–15.
IRVINGM., LOMBARDIV., PIAZZESIG. & FERENCZIM. A. (1992) Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature 357, 156–8.
JULIANF. J., SOLLINSK. R. & SOLLINSM. R. (1974) A model for the transient and steady-state mechanical behaviour of contracting muscle. Bioph. J. 14, 546–62.
KUSHMERICKM. J. & DAVIESR. E. (1969) The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Proc. R. Soc. London Ser. B 174, 315–53.
LOMBARDIV. & PIAZZESIG. (1990) The contractile response during steady lengthening of stimulated frog muscle fibres. J. Physiol. 431, 141–71.
LOMBARDIV., PIAZZESIG. & LINARIM. (1992) Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature 355, 638–41.
MATSUBARAI., YAGIN. & HASHIZUMEH. (1975) Use of an X-ray television for diffraction of the frog striated muscle. Nature 255, 728–9.
PATEE. & COOKER. (1989) A model of crossbridge action: the effects of ATP, ADP and Pi. J. Muscle Res. Cell Motility. 10, 181–96.
PIAZZESIG. & LOMBARDIV. (1995) A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 68, 1966–79.
PIAZZESIG., FRANCINIF., LINARIM. & LOMBARDIV. (1992) Tension transients during steady lengthening of tetanized muscle fibres of the frog. J. Physiol. 445, 659–711.
PIAZZESIG., LINARIM. & V.LOMBARDI (1993) In Mechanism of myofilament sliding in muscle contraction, (edited by SUGIH. & POLLACKG. H.) pp. 691–700. New York: Plenum Press.
RAYMENTI., HOLDENH. M., WHITTAKERM., YOHNC. B., LORENZM., HOLMESK. C. & MILLIGANR. A. (1993) Structure of the actin-myosin complex and its implications for musle contraction. Science 261, 58–65.
REEDYM. K., HOLMESK. C. & TREGEARR. T. (1965) Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature 207, 1276–80.
WOLEDGER. C., CURTINN. A. & HOMSHERE. (1985) Energetic aspects of muscle contraction. London: Academic Press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piazzesi, G., Lombardi, V. Simulation of the rapid regeneration of the actin-myosin working stroke with a tight coupling model of muscle contraction. J Muscle Res Cell Motil 17, 45–53 (1996). https://doi.org/10.1007/BF00140323
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00140323