Summary
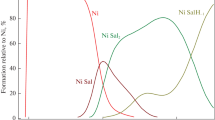
The complex formation equilibria involved in the binary and ternary systems of MII-SHAM and MII-SHAM-L [M = Cu, Ni, Co, Zn, Cd, Mn, Hg, Ca or UO2; SHAM = salicylhydroxamic acid; L = the ligating agent, N-(2-acetamido)iminodiacetic acid (ADA), iminodiacetic acid (IDA) or nitrilotriacetic acid (NTA)] were investigated by the potentiometric technique at 25 °C and ionic strength 0.1 m NaNO3. The results indicate the formation of 1∶1 and 1∶2 (metal ion:ligand) SHAM complexes. The formation of mixed-ligand complexes followed a stepwise mechanism, whereby an ML complex was first formed, followed by the ligation of SHAM. The relative stability of the mixed-ligand complex was compared with that of the binary complex. The concentration distribution of the complexes possibly formed in solution was evaluated. Solid complexes of SHAM were prepared and characterized by microanalysis, conductivity, i.r., electronic and n.m.r. spectroscopies, and magnetic susceptibility measurements.
Similar content being viewed by others
References
S. Piomelli and T. Loew, Hematol./Oncl. Clin N. Am., 5, 557 (1991).
M. S. El-Alfy, M. Mourad and S. Tawfik, Egypt J. Haematol., 13, 83 (1988).
A. S. Khalifa, T. H. Sallam, H. Ghaleb, B. J. Corden, A. A. Razek and M. Shoukry, Int. J. Pediatric Hematol./Oncl., 1, 315 (1994).
M. M. Khalil, A. H. H. Elghandour, M. Mostafa and M. M. Shoukry, Polyhedron, 13, 3295 (1994).
E. M. Shoukry, M. M. Khalil, A. H. H. Elghandour and M. M. Shoukry, Monatsh., 126, 241 (1995).
M. M. Shoukry, Bull.Soc. Chim. Fr., 130, 117 (1993).
M. M. Shoukry, W. M. Hosny and M. M. Khalil, Transition Met. Chem., 22, 252 (1995).
K. D. Hardman in H. Sigel (Ed.), Metal Ions in Biological Systems, Plenum Press, New York, 1973.
F. J. Welcher, The Analytical Uses of Ethylenediaminetetraacetic Acid, Van Nostrand, Princeton, 1965.
H. Gilman, Org. Syn. Coll., 1, 318 (1941).
R. G. Bates, Determination of pH-Theory and Practice, 2nd Edit., Wiley Interscience, New York, 1975.
M. M. Shoukry, M. M. Khater and E. M. Shoukry, Indian J. Chem., 25A, 488 (1986).
P. Gans, A. Sabatini and A. Vacca, Inorg. Chim. Acta, 18, 237 (1976).
R. B. Martin and R. J. J. Prados, J. Inorg. Nucl. Chem., 36, 1665 (1974).
L. Pettit, University of Leeds personal communication.
J. Geary, Coord. Chem. Rev., 7, 81 (1971).
M. M. Shoukry, K. Aziz, E. M. Shoukry and S. Hamdallah, Transition Met. Chem., 14, 115 (1989).
A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, Wiley, New York, 1980.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khairy, E.M., Shoukry, M.M., Khalil, M.M. et al. Metal complexes of salicylhydroxamic acid: equilibrium studies and synthesis. Transition Met Chem 21, 176–180 (1996). https://doi.org/10.1007/BF00136551
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00136551