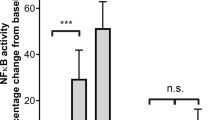
Recently we reported an antimetastatic activity of bacterial lipopolysaccharide (LPS) on a NK-cell-resistant murine fibrosarcoma (NFSa). Here we investigate and report the mechanistic significance of platelets in this activity. The number of circulating platelets was reduced to 63% of the control 3 days after an i.v. injection of 1.0 µg LPS, and then recovered to the level of control at day 10. Aggregation efficiency of platelets was impaired by LPS. The number of metastatic lung colonies after an i.v. injection of tumor cells was maximally reduced to 2.2% of the control at day 3 and increased in proportion to the recovery of platelet number. Neuraminidase (Ndase), which caused a non-immunological thrombocytopenia, also inhibited lung metastasis when injected prior to an i.v. tumor cell challenge. LPS and Ndase showed an identical pattern against five other syngeneic tumors; these agents inhibited lung metastases of the FSa fibrosarcoma and the SCC VII squamous cell carcinoma but failed to inhibit those of the NR-S1 squamous cell carcinoma, the MMCa#4 mammary adenocarcinoma and the NR-PG parotid gland tumor. All the three cells which were not responsive to any agents possessed a high aggregating activity of platelets while the other three tumors responsive to both agents did not show a detectable level of this activity. Platelet transfusion failed to modify the antimetastatic activity of LPS. These results suggest that platelets play an important role in the antimetastatic activity of LPS, though whether the role is principal or assistant remains to be seen.
Similar content being viewed by others
References
Jibu T, Ando K, Matsumoto T, Koike S, Kobori O, Morioka Y and Kanegasaki S, 1991, Active components of intestinal bacteria for abdominal irradiation-induced inhibition of lung metastasis. Clinical & Experimental Metastasis, 9, 529–540.
Jibu T, Koike S, Ando K, Matsumoto T, Kimoto M and Kanegasaki S, 1993, Antimetastatic activity of lipopolysaccharide against a NK-resistant murine fibrosarcoma. Clinical & Experimental Metastasis, in press.
Stenberg PE, Levin J, Baker G, Mok Y and Corash L, 1991, Neuraminidase-induced thrombocytopenia in mice: effects on thrombopoiesis. Journal of Cellular Physiology, 147, 7–16.
Jones D, Wallace A and Fraser E, 1971, Sequence of events in experimental metastasis of Walker 256 tumor: light immunofluorescent and electron microscopic observation. Journal of the National Cancer Institute, 46, 493–504.
Gasic GJ, Gasic TB, Galanti N, Johnston T and Murphy S, 1973, Platelet-tumor cell interaction in mice. The role of platelets in the spread of malig nant disease. International Journal of Cancer, 11, 704–718.
Pareti FI, Capitanio A and Mannucci PM, 1976, Acquired storage pool disease in platelets during disseminated intravascular coagulation. Blood, 48, 511–515.
Weil MH, Spink WW, 1957, A comparison of shock due to endotoxin with anaphylactic shock. Journal of Laboratory and Clinical Medicine, 50, 501–515.
McIntire FC, Sievert HW, Barlow GH, Finley RA and Lee AY, 1976, Chemical, physical and biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry, 6, 2363–2372.
Kanegasaki S, Tanamoto K, Yasuda T, Homma J, Matsuura M, Nakatsuka M, Kumazawa Y, Yamamoto A, Shiba T, Kusumoto S, Imoto M, Yoshimura mH and Shimamoto T, 1986, Structureactivity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid composition. Journal of Biochemistry, 99, 1230–1210.
Galanos C, Rietschel ET, Lüderitz O and Westphal O, 1972, Biological activities of lipid A complexed with bovine-serum albumin. European Journal of Biochemistry, 31, 230–233.
Matsumoto T, Ando K and Koike S, 1991, Significance of bacterial flora in abdominal irradiationinduced inhibition of lung metastasis. Cancer Research, 48,3031–3034.
Mogi Y, Kogawa K, Takayama T, Yoshizaki N, Bannai K, Muramatsu H, Koike K, Kohgo Y, Watanabe N and Nütsu Y, 1991, Platelet aggregation induced by adenosin diphosphate released from cloned murine fibrosarcoma cells positively correlated with the experimental metastatic potential of the cells. Japanese Journal of Cancer Research, 82, 192–198.
Nütsu Y, Ishigaki S, Kogawa K, Mogi Y, Watanabe N, Kohogo Y and Urushibara I, 1988, Effect of combined administration of prostacyclin analogue and adriamycin against the artificial metastasis of Meth A cell. Invasion and Metastasis, 8, 57–72.
Takayama K, Qureshi N, Beutler B and Kirkland K, 1989, Diphosphoryl lipid A from Rodopseudomonus sphaeroides ATCC1702 blocks induction of cachectin in macrophages by lipopolysaccharide. Infection and Immunity, 57, 1336–1338.
Morrison DC and Ulevitch RJ, 1978, The effect of bacterial endotoxins on host mediation systems. American Journal of Pathology, 93, 527–618.
WestphalO, 1974, Bacterial endotoxins. International Archives of Allergy and Applied Immunology, 49, 1–43.
Saiki I, Maeda H, Murata J, Yamamoto N, Kiso M, Hasegawa A and Azuma I, 1989, Antimetastatic effect of endogenous tumor necrosis factor induced by the treatment of recombinant interferon y followed by an analogue (GLA-4) to synthetic lipid A subunit. Cancer Immunology and Immunotherapy, 30, 151–157.
Dustin ML and Springer TA, 1988, Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. Journal of Cell Biology, 107, 321–331.
Springer TA, 1990, Adhesion receptors of the immune system. Nature, 346, 425–434.
Fidler IJ and Hart IR, 1982, Biological diversity in metastatic neoplasms: origin and implications. Science, 217, 998–1003.
Hanna N and Fidler IJ, 1980, Role of natural killer cells in the destruction of circulating tumor emboli. Journal of the National Cancer Institute, 65, 801–809.
Hanna N, 1982, Inhibition of experimental tumor metastasis by selective activation of natural killer cells. Cancer Research, 42, 1337–1342.
Gasic GJ, Gasic TB and Stewart CC, 1968, Antimetastatic effects associated with platelet reduction. Proceedings of the National Academy of Sciences, USA, 61, 46–52.
Diana HA and John D, 1978, Endotoxin-induced changes in human platelet membranes: morphologic evidence. Blood, 51, 487–495.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kimoto, M., Ando, K., Koike, S. et al. Significance of platelets in an antimetastatic activity of bacterial lipopolysaccharide. Clin Exp Metast 11, 285–292 (1993). https://doi.org/10.1007/BF00121171
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00121171