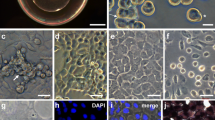
The zebrafish is a popular model for studies of vertebrate development and toxicology. However, in vitro approaches with this organism have not been fully exploited because cell culture systems have been unavailable. We developed methods for the culture of cells from blastula-stage diploid and haploid zebrafish embryos, as well as cells from the caudal and pelvic fin, gill, liver, and viscera of adult fish. The haploid embryo-derived cells differentiated in culture to a pigmented phenotype and expressed, upon exposure to 2,3,7,8-tetrachlorodibenzo p-dioxin, a protein that was immunologically and functionally similar to rainbow trout cytochrome P450IA1 Zebrafish cultures were grown in a complex basal nutrient medium supplemented with insulin, trout embryo extract, and low concentrations of trout and fetal bovine serum; they could not be maintained in conventional culture medium containing a high concentration of mammalian serum. Using calcium phosphate-mediated transfection, a plasmid constructed for use in mammalian cells was introduced into zebrafish embryo cell cultures and expressed in a stable manner. These results indicated that the transfection procedures utilized in mammalian systems can also be applied to zebrafish cell cultures, providing a means for in vitro alteration of the genotype and phenotype of the cells.[/ p]
Similar content being viewed by others
Abbreviations
- TCDD,:
-
2,3,7,8-tetrachlorodibenzo-p-dioxin
- EROD,:
-
7-ethyoxyresorufin
- HDPDS,:
-
4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid
- EDTA,:
-
ethylanediaminetetraacetic acid
- FBS,:
-
fetal bovine serum
- LDF,:
-
limit dilution factor
- DMSO,:
-
dimethyl sulfoxide
- ES,:
-
embryonal stem
- PAH,:
-
polycylic aromatic hydrocarbons
- ZG,:
-
zebrafish gill
- ZBF,:
-
zebrafish pelvic fin
- ZV,:
-
Zebrafish viscera
- ZCF,:
-
zebrafish caudal fin
- ZEM,:
-
diploid blastula-derived
References
AOKI, K., and MATSUDAIRA, H. (1977). “Induction of hepatic tumors in a teleost (Oryzias latipes) after treatment with methylazoxymethanol acetate.” J. Natl. Cancer Inst. 59: 1747–1749.
BABICH, H., and BORENFREUND, E. (1991). “Cytoxicity and genotoxicity assays with cultured fish cells: a review.” Toxic.In Vitro 5: 91–100.
BAILEY, G.S., HENDRICKS, J.D., NIXON, J.E., and PAWLOWSKI, N.E. (1984). “The sensitivity of rainbow trout and other fish to carcinogens.” Drug Metab. Rev. 15: 725–750.
BOLS, N.C., and LEE, L.E.J. (1991). “Technology and uses of cell cultures from the tissues and organs of bony fish.” Cytotechnology 6: 163–188.
BRADFORD, M.M. (1976). “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.” Anal. Biochem. 72: 248–254.
BRESCH, H. (1991). “Early life-stage test in zebrafish versus a growth test in rainbow trout to evaluate toxic effects.” Bull. Environ. Contam. Toxicol. 46: 641–648.
BRESCH, H., BECK, H., EHLERMANN, D., SCHLASZUS, H., and URBANEK, M. (1990). “A longterm toxicity test comprising reproduction and growth of zebrafish under 4-chloroaniline.” Arch. Environ. Contam. Toxicol. 19: 419–427.
Cupollodi, P., Kupamei, Y., Supharps, A., Wupeber, D., and Buparnes, D. (1992a). “Fish embryo cell cultures for derivation of stem cells and transgenic chimeras.” Marine Mol. Biol. and Biotech. (in press).
Cupollodi, P., Mupiranda, C., Zuphao, X., Bupuhler, D., and Buparnes, D. (1992b). “Induction of cytochrome P450 in zebrafish cells in culture by 2,3,7,8-tetrachlorodibenzo-p-dioxin.” The Toxocologist (in press).
COLLODI, P., and BARNES, D.W. (1990). “Mitogenic activity from trout embryos.” Proc. Natl. Acad. Sci. 87: 3498–3502.
COLLODI, P., STEKOLL, M.S., and RICE, S.D. (1984). “Hepatic aryl hydrocarbon hydroxylase activities in coho salmon (Oncorhynchus kisutch) exposed to petroleum hydrocarbons.” Comp. Biochem. Physiol. 79C: 337–341.
DAVE, G., and XIU, R. (1991). “Toxicity of mercury, copper, nickel, lead and cobalt to embryos and larvae of zebrafish,Brachydanio rerio.” Arch. Env. Cont. Tox. 21: 126–134.
ENDO, A., and INGALLS, T.H. (1968). “Chromosomes of the zebrafish.” J. of Heredity 59: 382–384.
ERNST, T., JACKSON, C., and BARNES, D. (1991). “Karyotypic stability of serum-free mouse embryo cells.” Cytotechnology 5: 211–222.
EGAMI, N., KYONO-HAMAGUCHI, Y., MITANI, H., and SHIMA, A. (1981). “Characteristics of hepatoma produced by treatment with diethylnitrosamine in the fish,Oryzias latipes.” In: Phyletic Approaches to Cancer (C.J. Dawe, ed.), Japan Scientific Press, Tokyo. pp 217–226.
EVANS, M.J. and KAUFMAN, M.H. (1981). “Establishment in culture of pluripotential cells from mouse embryos.” Nature 292: 154–156.
GOKSOYR, A., ANDERSSON, T., BUHLER, D.R., Sputegeman, J.J., WILLIAMS, D.E., and FORLIN, L. (1991). “Immunochemical cross-reactivity of beta-naphthoflavone-inducible cytochrome P450 (P4501A) in liver microsomes from different fish species and rat.” Fish Physiol. Biochem. 9: 1–13.
HATANAKA, J., DOKE, T., HIRADA, T., AIKAWA, T., and ENOMOTO, M. (1982), “Usefulness and rapidity of screening for the toxicity and carcinogenicity of chemicals in medaka,Oryzias latipes.” Jpn. J. Exp. Med. 52: 243–253.
HELMRICH, A., BAILEY, G., and BARNES, D. (1988). “Transfection of cultured fish cells with exogenous DNA.” Cytotech. 1: 215–222.
HENDRICKS, J.D., MEYERS, T.R., CASTEEL, J.L., NIXON, J.E., LOVELAND, P.M., and BAILEY, G.S. (1984). “Rainbow trout embryos: Advantages and limitations for carcinogenesis research.” Natl. Cancer Inst. Monogr. 65: 129–137.
ISHINO, F., KANEKO-ISHINO, T., ITO, M., MATSUHASHI, M., YOKOYAMA, M., and KATSUKI, M. (1990). “Developmental potential of haploid-derived parthenogenetic cells in mouse chimeric embryos.” Develop. Growth & Differ. 32: 139–144.
KAUFMAN, M.H., ROBERTSON, E.J., HANDYSIDE, A.H., and EVANS, M.J. (1983). “Establishment of pluripotential cell lines from haploid mouse embryos.” J. Embryol. Exp. Morphol. 73: 249–258.
KELLEY, M., HANTELLE, P., SAFE, S., LEVIN, W., and THOMAS, P.E., (1987). “Co-induction of cytochrome P-450 enzymes in rat liver of 2,4,5,2′, 4′,5′-hexachlorobiphenyl or 3-methoxy-4-aminoazobenzene.” Mol. Pharmacol. 32: 206–211.
KIMMEL, C.B., and WARGA, R.M. (1986). “Tissue-specific cell lineages originate in the gastrula of the zebrafish.” Science 231: 365–368.
LOO, D., RAWSON, C., HELMRICH, A., and BARNES, D. (1989). “Serum-free mouse embryo (SFME) cells: growth responsesin vitro.” J. Cell. Physiol. 139: 484–491.
LOO, D., FUQUAY, J.I., RAWSON, C., and BARNES, D.W. (1987). “Extended culture of mouse embryo cells without senescence: inhibition by serum.” Science 236: 200–202.
LOWRY, O.H., ROSEBROUGH, N.J., FARR, A.L., and RANDALL, R.J. (1951). “Protein measurement with the Folin phenol reagent.” J. Biol. Chem. 193: 265–275.
NAGEL, R., BRESCH, H., CASPERS, N., HANSEN, P.D., MARKERT, M., MUNK, R., SCHOLZ, N., and TER HOFTE, B.B. (1991). “Effect of 3,4-dichloroaniline on the early life stages of the zebrafish (Brachydanio rerio): results of a comparative laboratory study.” Ecotox. Environ. Safety 21: 157–164.
NANDI, A.K., ROGINSKI, R.S., GREGG, R.G., SMITHIES, O., and SKOULTCHI, A.I. (1988). “Regulated expression of genes inserted at the human chromosomal beta-globulin locus by homologous recombination.” Proc. Natl. Acad. Sci. 85: 3845–3849.
PESONEN, M., GOKSOYR, A., and ANDERSSON, T. (1989). “Induction of xenobiotic metabolizing enzyme activities in primary culture of rainbow trout hepatocytes.“ Marine Environ. Res. 28: 113–116.
POTTENGER, L.H., and JEFCOATE, C.R. (1990).“Characterization of a novel cytochrome P450 from the transformable cell line, C3H/10Tl/2.” Carcinogenesis 11: 321–327.
PROUGH, R.A., BURKE, M.D., and MAYER, R.T. (1978). “Direct fluorometric methods for measuring mixed-function oxidase activity.” Methods Enzymol. 52: 372–377.
RAWSON, C., LOO, D., HELMRICH, A., ERNST., T. NATSUNO, T., MERRILL, G., and BARNES, D. (1991). “Serum inhibition of proliferation of serum-free mouse embryo (SFME) cells.” Exp. Cell Res. 192: 271–277.
ROBERTSON, E., BRADLEY, A., KUEHN, M., and EVANS, M. (1986). “Germ-line transmission of genes introduced into culture pluripotential cells by retroviral vector.” Nature 323: 445–448.
SCHULTZ, M.E., and SCHULTZ, R.J. (1982a). “Diethylnitrosamine-induced hepatic tumors in wild vs. inbred strains of a viviparous fish.” J. Heredity 73: 43–48.
SCHULTZ, M.E., and SCHULTZ, R.J. (1982b). “Induction of hepatic tumors with 7,12-dimethylbenz[a]anthracene in two species of viviparous fishes (genus Poeciliopsis).” Environ. Res. 27: 337–351.
SHIRAHATA, S., RAWSON, C., LOO, D., CHANG, Y., and BARNES, D. (1990). “Ras andneu oncogenes reverse serum inhibition and epidermal growth factor dependence of serum-free mouse embryo (SFME) cells.” J. Cell. Physiol. 144: 69–76.
SOUTHERN, P.J., and BERG, P. (1982). “Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter.” J. Mol. and App. Genetics 1: 327–341.
STREISINGER, G., WALKER, C., DOWER, N., KNAUBER, D., and SINGER, F. (1981). “Production of clones of homozygous diploid zebrafish (Brachydanio rerio).” Nature 291: 293–296.
STUART, G.W., McMURRAY, J.V., and WESTERFIELD, M. (1988). “Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos.” Development 103: 403–412.
STUART, G.W., VIELKIND, J.R., McMURRAY, J.V., and WESTERFIELD, M. (1990). “Stable lines of transgenic zebrafish exhibit reproducible patterns of transgenic expression.” Development 109: 577–584.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collodi, P., Kame, Y., Ernst, T. et al. Culture of cells from zebrafish (Brachydanio rerio) embryo and adult tissues. Cell Biol Toxicol 8, 43–61 (1992). https://doi.org/10.1007/BF00119294
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00119294