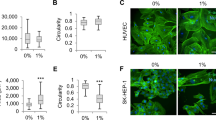
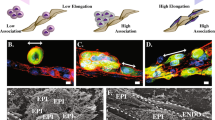
Bovine capillary endothelial cells (BCEC), cultured in suspension on a rotary shaker, formed aggregates ranging from 50 to 300 µm in diameter. In plasma clot these aggregates sprouted in multiple directions and gave rise to vascular channels. Aggregates of the squamous cell carcinoma line of rat bladder NBT-II-81, cultured in plasma clot, formed solid spheroids that grew slowly by expansion. When cultured together with BCEC, however, NBT-I I-81 infiltrated the plasma clot extensively. The tumor cells, after establishing contacts with the vascular channels, spread into the fibrin meshwork using the subendothelial space as their path of propagation. Endothelial cells that were separated from the surrounding matrix by invading tumor cells degenerated, leaving behind channels lined only by neoplastic epithelium. The adhesive properties of the subendothelial matrix were studied by seeding NBT-I I-81 cells on dishes coated with the extracellular matrix produced by BCEC. Tumor cells attached readily and in large numbers to dishes coated with the subendothelial matrix. In contrast they attached poorly to dishes coated with fibrin. We conclude that the spread of carcinoma cells into plasma clot is markedly enhanced by endothelial channels, developed in the absence of blood flow. The production of a highly adhesive extracellular matrix by the capillary endothelium during angiogenesis may represent an important element in the preferential growth of the tumor along the vascular route.
Similar content being viewed by others
References
Ausprunk, D. H., 1982, Synthesis of glycoproteins by endothelial cells in embryonic blood vessels. Development Biology, 90, 79–90.
Clark, R. A., Della Pelle, P. D., Manseau, E., Lanigan, J. M., Dvorak, H. F., and Colvin, R. B., 1982, Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. Journal of Investigative Dermatology, 79, 269–276.
Folkman, J., and Hochberg, M., 1973, Self regulation of growth in three dimensions. Journal of Experimental Medicine, 138, 745–753.
Folkman, J., 1974, Tumor angiogenesis factor. Cancer Research, 34, 2109–2113.
Folkman, J., 1975, Tumor angiogenesis. Cancer Biology of Tumors, edited by F. F. Becker (New York: Plenum Press), pp. 355–388.
Furcht, L. T., McCarthy, J. B., Palm, S. L., Basara, M. L., and Enenstein, J., 1984, Peptide fragments of laminin and fibronectin promote migration (haptotaxis and chemotaxis) of metastatic cells. Basement Membranes and Cell Movement, edited by R. Porter and J. Whelan (London: Pitman), pp. 130–145.
Gimbrone, M. A., Jr., Leapman, S., Cottran, R. S., and Folkman, J., 1972, Tumor dormancy in vivo by prevention of neovascularization. Journal of Experimental Medicine, 136, 261–276.
Gospadarowicz, D., and Greenberg, D., 1981, The role of growth factors and extracellular matrices in the control of mammalian cell proliferation. The Biology of Normal Human Growth, edited by A. Aperia, K. Hall, A. Larsson, A. Zetterberg and R. Zetterstrom (New York: Raven Press), pp. 11–21.
Gospodarowicz, D., Greenburg, G., Foidart, J. M., and Savion, N., 1981, The production and localization of laminin in cultured vascular and corneal endothelial cells. Journal of Cell Physiology, 107, 171–183.
Humphreys, W. J., Sposlock, B. O., and Johnson, J. S., 1974, Critical point crying of ethanol infiltrated cryofractured biological specimens for scanning electron microscopy. Scanning Electron Microscopy/1974, 275–282.
Jaffe, E. A., and Mosher, D. F., 1978, Synthesis of fibronectin by cultured human endothelial cells. Journal of Experimental Medicine, 147, 1779–1791.
Kramer, R. H., Gonzalez, R., and Nicholson, G., 1980, Metastatic tumor cells adhere preferentially to the extracellular matrix underlying vascular endothelial cells. International Journal of Cancer, 26, 639–645.
Kramer, R. H., Vogel, K. G., and Nicolson, G. L., 1982, Tumor cell interactions with vascular endothelial cells and their extracellular matrix. Interactions of Platelets and Tumor Cells, edited by G. A. Jamieson (New York: Liss), pp. 333–351.
Lacovara, J., Cramer, E. B., and Quigley, J. P., 1984, Fibronectin enhancement of directed migration of B16 melanoma cells. Cancer Research, 44, 1657–1663.
Leighton, J., Tchao, R., Stein, R., and Abaza, N., 1980, Histophysiologic gradient culture of stratified epithelium. Normal human tissue and cell culture. Methods in Cell Biology, Vol. 21B, edited by C. C. Harris, B. F. Trump, G. D. Stoner (New York: Academic Press), pp. 287–307.
Leighton, J., Tchao, R., Nicosia, R. F., and Schroyens, W., 1983, Analysis of some tissue processes involved in the propagation of cancer using histophysiologic gradient culture. 13th International Cancer Congress, Part C, Biology of Cancer (2) (New York: Liss), pp. 51–62.
Liotta, L., Rao, C. N., and Barsky, S. H., 1983, Tumor invasion and the extracellular matrix. Laboratory Investigation, 49, 636–649.
Moscona, A. A., 1961, Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interaction in vitro. Experimental Cell Research, 22, 455–475.
Nicolson, G. L., Izimura, T., Gonzalez, R., and Ruoslathl, E., 1981, The Role of fibronectin in the adhesion of metastatic melanoma cells to endothelial cells and their basal lamina. Experimental Cell Research, 135, 461–465.
Nicosia, R. F., Tchao, R., and Leighton, J., 1982, Histotypic angiogenesis in vitro: light microscopic, ultrastructural and radioautographic studies. In Vitro, 18, 538–549.
Nicosia, R. F., Tchao, R., and Leighton, J., 1983, Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Research, 43, 2159–2166.
Reynolds, E. S., 1963, The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Journal of Cell Biology, 17, 208–212.
Tannock, I. F., 1983, Biology of tumor growth. Hospital Practice, 18, (No. 4), 81–93.
Tchao, R., 1982, Novel forms of epithelial cell motility on collagen and on glass surfaces. Cell Motility, 4, 333–341
Vlodavsky, I., and Gospodarowicz, D., 1981, Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature, 289, 304–306.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicosia, R.F., Tchao, R. & Leighton, J. Interactions between newly formed endothelial channels and carcinoma cells in plasma clot culture. Clin Exp Metast 4, 91–104 (1986). https://doi.org/10.1007/BF00119076
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00119076