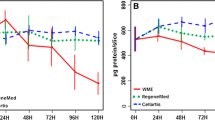
Rat hepatocytes prepared by collagenase digestion or ED TA dissociation were examined in culture for comparison of culture stability and morphology, and retention of selected adult rat liver characteristics. Cells prepared by EDTA perfusion followed by Percoll cen trifugation were deemed to form confluent monolayer cultures more rapidly and monolayers remained intact for up to 21 days without signs of nonparenchymal cell growth or loss of primary hepatocyte appearance. The spectrally determined cytochrome P-450 content remained constant through eight days in culture. Collagenase prepared cells contained an identical amount of P-450 but within 72 hr lost greater than 80% of the spectrally detectable P-450. Glutathione (GSH) content was higher in the EDTA-prepared hepatocytes and remained constant with only a modest effect of transferrin and selenium (TI S) supplementation, while GSH levels in collagenaseprepared cells increased, thereafter decreased with time in culture and was dependent on TI S supplementation. Cells prepared with ED TA also displayed an increase in GSH efflux rate in response to chronic GSH depletion by ethacrynic acid. γ-Cystathionase (CNase) activity was retained at initial levels in ED TAprepared hepatocytes supplemented with TI S and declined only about 25% in unsupplemented cells. Collagenase-prepared cells lost 75% of CNase activity by 72 hr. The established marker of hepatocyte neoplastic transformation, γ-glutamyl trans-peptidase (GGT), increased rapidly in collagenase-prepared cells. The accumulation of GGT was slowed by T/S supplementation. GGT activity did not increase in EDTA-prepared hepatocytes. Evaluation of morphological and biochemical criteria suggest that hepatocytes prepared without collagenase present superior model systems for the study of biochemical events through more extended culture times.
Similar content being viewed by others
Abbreviations
- CNase:
-
γ-cystathionase
- EDTA:
-
ethylenediamine tetraacetic acid
- GGT:
-
γ-glutamyl transpeptidase
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- HEPES:
-
N-2-hydroxyethylpiperizine-N′-2-ethane-sulfonic acid
- SAH:
-
S-adenosylhomocysteine
- SAM:
-
S-adenosylmethionine
- T/S:
-
transferrin- and selenite-supplemented
References
AW. T.K., OOKHTENS, M. and KAPLOWITZ, N. (1986). Mechanism of inhibition of glutathione efflux by methionine from isolated rat hepatocytes. Am. J. Physiol. 251: G354-G361.
BARNES, D. and SATO, G. (1980). Serum-free cell culture: a unifying approach. Cell 22:649–653.
BERRY, M.N., FARRINGTON, C., GAY, S., GRIVELL, A.R. and WALLACE, P.G. (1983). Preparation of isolated hepatocytes in good yield without enzymatic digestion. In: Isolation, Characterization, and Use of Hepatocytes (R.A. Harris and N. W. Cornell, eds.), pp. 7–10. Elsevier Science Publishing Co., Inc., New York.
BERRY, M.N. and FRIEND, D.S. (1969). High yield preparation of isolated rat parenchymal cells. A biochemical and fine structure study. J. Cell Biol. 43:506–520.
BOREK, C. and WILLIAMS, G.M. (Eds.). (1980). Differentiation and Carcinogenesis in Liver Cell Cultures. Ann. N.Y. Acad. Sci. Vol. 349.
COOK, R.J. and WAGNER, C. (1984). Glycine N-methyltransferase is a folate binding protein in rat liver cytosol. Proc. Natl. Acad. Sci. U.S.A. 81:3631–3634.
CRABB, D.W. and ROEPKE, J. (1987). Loss of growth hormone-dependent characteristics of rat hepatocytes in culture. In Vitro Cell. Develop. Biol. 23:303–307.
DALET, C., FEHLMANN, M. and DEBET, P. (1982). Use of Percoll density gradient centrifugation for preparing isolated rat hepatocytes having long-term viability. Anal. Biochem. 122:119–123.
EDWARDS, A. M. (1982). Regulation of γ-glutamyl transpeptidase in rat hepatocyte monolayer cultures. Cancer Res. 42:1107–1115.
FARISS, M.W. and REED, D.J. (1983). Measurement of glutathione and glutathione disulfide efflux from isolated rat hepatocytes. In: Isolation, Characterization, and Use of Hepatocytes (R.A. Harris and N.W. Cornell, eds.), pp. 349–355. Elsevier Science Publishing Co., Inc., New York.
HARGROVE, J.L. and WICKMAN, R.D. (1987).A cystine-dependent inactivator of tyrosine aminotransferase co-purifies with γ-cystathionase (cysteine desulfurase). J. Biol. Chem. 262:7351–7357.
HOGBERG, J. and KRISTOFSEN, A. (1977).A correlation between glutathione levels and cellular damage in isolated hepatocytes. Eur. J. Biochem. 74:77–82.
INGLEHART, J.D., YORK, R.M., MODEST, A.P., LAZARUS, M. and LIVINGSTON, D. M. (1977). Cysteine requirement of continuous human lymphoid cell lines of normal and leukemic origin. J. Biol. Chem. 252:7184–7191.
JONES, P.A. (1986). DNA methylation and cancer. Cancer Res. 46:461–466.
KREAMER, B.L., STRAEKER, J.L., SAWADA, N., SATTLER, G.L., HSIA, M.T.S. and PITOT, H.C. (1986). Use of low-speed iso-density Percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell. Devel. Biol. 22:201–211.
MEISTER, A., TATE, S.S. and GRIFFITH, O.W. (1981). γ-Glutamyl transpeptidase. Methods Enzymol. 77:237–253.
MEREDITH, M.J. (1986). Time in culture alters the glutathione content and sensitivity to ethacrynic acid of rat hepatic monolayer cultures. Cell Biol. Toxicol. 2:495–505.
MEREDITH, M.J. (1987). Cystathionase activity and glutathione metabolism in redifferentiating rat hepatocyte primary cultures. Cell Biol. Toxicol. 3:361–377.
MEREDITH, M.J. and WILLIAMS, G.M. (1986). Intracellular glutathione cycling by γ-glutamyl transpeptidase in tumorigenic and nontumorigenic cultured rat liver cells. J. Biol. Chem. 261:4986–4992.
NEWMAN, S. and GUZELIAN, P. (1982). Stimulation of de novo synthesis of cytochrome P-450 by phenobarbital in primary nonproliferating cultures of adult rat hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 79:2922–2926.
OMURA, T. and SATO, R. (1964). The carbon monoxide-binding pigment of liver microsomes II. Solubilization, purification and properties. J. Biol. Chem. 239:2379–2385.
REED, D.J., BABSON, J.R., BEATTY, P.W., BRODIE, A.E., ELLIS, W.W. and POTTER, D.W. (1980). High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 106:55–62.
SEGLEN, P.O. (1972). Preparation of rat liver cells. II. Effects of ions and chelators on tissue dispersion. Exp. Cell. Res. 76:25–30.
SHANK, R.C. (1984). Toxicity-induced aberrant methylation of DNA and its repair. Pharm. Rev. 36:19S–24S.
SMITH, P.K., KROHN, R.I., HERMANSON, G.T., MALLIA, A.K., GARTER, F.H., PROVENZANO, M.D., FUJIMOTO, E.K., GOEKE, N.M., OLSON, B.J. and KLENK, D.C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85.
STEWARD, R.A., DANNAN, G.A., GUZELIAN, P.S. and GUENGERICH, F.P. (1985). Changes in the concentration of seven forms of cytochrome P-450 in primary cultures of adult rat hepatocytes. Mol. Pharmacol. 27:125–132.
STROM, S.C., JIRTLE, R.L., JONES, R.S., NOVICKI, D.L., ROSENBERG, M.R., NOVOTNY, A., IRONS, G., MCLAIN, J.R. and MICHALOPOULOS, G. (1982). Isolation, culture and transplantation of human hepatocytes. J. Natl. Cancer Inst. 68:771–778.
WAGNER, C., BRIGGS, W.T. and COOK, R.J. (1985). Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem. Biophys. Res. Commun. 127:746–752.
WANG, S-R., RENAUD, G., INFANTE, J., CATALA, D. and INFANTE, R. (1985). Isolation of rat hepatocytes with EDTA and their metabolic functions in primary culture. In Vitro Cell. Develop. Biol. 21:526–530.
WEST, D.C., SATTAR, A. and KUMAR, S. (1985). A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal. Biochem. 147:289–295.
WILLIAMS, G.M. and GUNN, J.M. (1974). Long-term culture of adult rat liver epithelial cells. Exp. Cell Res. 89:139–142.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meredith, M.J. Rat hepatocytes prepared without collagenase: Prolonged retention of differentiated characteristics in culture. Cell Biol Toxicol 4, 405–425 (1988). https://doi.org/10.1007/BF00117769
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00117769