Abstract
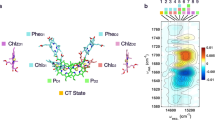
We have measured the rate constant for the formation of the oxidized chlorophyll a electron donor (P680+) and the reduced electron acceptor pheophytin a − (Pheo a −) following excitation of isolated Photosystem II reaction centers (PS II RC) at 15 K. This PS II RC complex consists of D1, D2, and cytochrome b-559 proteins and was prepared by a procedure which stabilizes the protein complex. Transient absorption difference spectra were measured from 450–840 nm as a function of time with 500fs resolution following 610 nm laser excitation. The formation of P680+-Pheo a − is indicated by the appearance of a band due to P680+ at 820 nm and corresponding absorbance changes at 490, 515 and 546 nm due to the formation of Pheo a −. The appearance of the 490 nm and 820 nm bands is monoexponenital with τ=1.4±0.2 ps. Treatment of the PS II RC with sodium dithionite and methyl viologen followed by exposure to laser excitation results in accumulation of Pheo a −. Laser excitation of these prereduced RCs at 15 K results in formation of a transient absorption spectrum assigned to 1*P680. We observe wavelength-dependent kinetics for the recovery of the transient bleach of the Qy absorption bands of the pigments in both untreated and pre-reduced PS II RCs at 15K. This result is attributed to an energy transfer process within the PS II RC at low temperature that is not connected with charge separation.
Similar content being viewed by others
Abbreviations
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- RC:
-
reaction center
- P680:
-
primary electron donor in Photosystem II
- Chl a :
-
chlorophyll a
- Pheo a :
-
pheophytin a
References
Allen JP, Feher G, Yeates T-O, Komiya H and Rees DC (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26: The protein subunits. Proc Natl Acad Sci USA 84: 6162–6166
Barber J, Chapman DJ and Telfer A (1987) Characterization of a PSII Reaction Center isolated from the chloroplasts of Pisum Sativum. FEBS Lett. 220: 67–73
Bixon M and Jortner J (1986) Coupling of protein modes to electron transfer in bacterial photosynthesis. J Phys Chem 90: 3795–3800
Breton J, Martin JL, Migus A, Antonetti A and Orszag A (1986a) Femtosecond spectroscopy of excitation energy transfer and intial charge separation in the reaction center of the photosynthetic bacterium Rhodopseudomonas viridis. Proc Natl Acad Sci USA 83: 5121–5125
Breton J, Martin JL, Migus A, Antonetti A (1986b) The absence of a spectroscopically resolved intermediate state P+B− in bacterial photosynthesis. FEBS Lett 209: 37–43
Breton J, Martin J-L, Fleming GR and Lambry J-C (1988) Low temperature femtosecond spectroscopy of the initial step of electron transfer in reaction centers from photosynthetic purple bacteria. Biochemistry 27: 8276–8284
Chang C-H, Tiede DM, Tang J, Smith U, Norris J and Schiffer M (1986) Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett 205: 82–86
Davis MS, Forman A and Fajer J (1979) Ligated chlorophyll cation radicals: Their function in photosystem II of plant photosynthesis. Proc Natl Acad Sci USA 76: 4170–4174
Deisenhofer J, Epp O, Miki K, Huber R and Michel H (1984) X-ray structure analysis of a membrane protein complex. Electron density map at 3Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol 180: 385–398
Deisenhofer J, Epp O, Miki K, Huber R and Michel H (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature (London). 318: 618–624
DeVault D (1984) Quantum mechanical tunnelling in biological systems. Cambridge: Cambridge University Press
Dunahay TG, Staehelin LA, Seibert M, Ogilvie PD and Berg SP (1984) Structural, biochemical, and biophysical characterization of four oxygen-evolving photosystem II preparations from spinach. Biochim Biophys Acta 764: 179–193
Erickson JM, Rochaix JD and Delepelaire P (1985) In: Molecular Biology of the Photosynthetic Apparatus, pp 53–65. Cold Spring Harbor Laboratory
Fleming GR, Martin JL and Breton J (1988) The primary electron transfer in photosynthetic bacteria. Nature 333: 190–192
Fujita I, Davis MS and Fajer J (1978) Anion radicals of pheophytin and chlorophyll a: Their role in the primary charge separations of plant photosynthesis. J Am Chem Soc 100: 6280–6282
Grinvald A (1976) The use of standards in analysis of fluorescence decay data. Anal Biochem 75: 260–280
Holten D and Kirmaier C (1987) Primary photochemistry of reaction centers from the photosynthetic purple bacteria. Photosynth Res 13: 225–260
Ikeuchi M and Inoue Y (1988a) A new 4.8 kDa polypeptide intrinsic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low molecular weight proteins. Plant Cell Physiol 29: 1233–1239
Ikeuchi M and Inoue Y (1988b) A new photosystem II reaction center component (4.8 kDa protein) encoded by chloroplast genome. FEBS Lett 241: 99–104
Jankowiak R and Small GJ (1987) Hole-burning spectroscopy and relaxation dynamics of amorphous solids at low temperature. Science 237: 618–625
Jankowiak R, Tang D, Small GJ and Seibert M (1989) Transient and persistent hole burning of the reaction center of PS II. J Phys Chem 93: 1649–1654
Kirmaier C, Holten D and Parson WW (1985) Temperature and detection-wavelength dependence of the picosecond electron transfer kinetics measured in Rhodopseudomonas sphaeroides reaction centers. Resolution of new spectral and kinetic components in the primary charge separation process. Biochim Biophys Acta 810: 33–48
Kirmaier C, Holten D and Parson WW (1986a) Picosecond study of the P+I−Q→IQ− electron transfer reaction in Rps. viridis reaction centers. Biophys J 49: 586a
Kirmaier C, Blankenship RE and Holten D (1986b) Formation and decay of radical-pair state P+I− in Chloroflexus aurantiacus reaction centers. Biochim Biophys Acta 850: 275–285
Klimov VV, Allakhverdiev SI, Demeter S and Krasnovskii AA (1979) Dokl Akad Nauk SSSR 249: 227–230
Martin J-L Breton J, Hoff A, Migus A and Antonetti A (1986) Femtosecond spectroscopy of the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin with a time constant of 2.8 ps 0.2 ps. Proc Natl Acad Sci USA 83: 957–961
McTavish H, Picorel R and Seibert M (1989) Stabilization of isolated PS II reaction center complex in the dark and in the light using polyethylene glycol. Plant Physiol 89: 452–456
Mimuro M, Yamazaki I, Itoh S, Tamai N and Satoh K (1988) Dynamic fluorescence properties of D1−D2-cytochrome b−559 complex isolated from spinach chloroplasts: Analysis by means of the time-resolved fluorescence spectra in picosecond time range. Biochim Biophys Acta 933: 478–486
Nanba O and Satoh K (1987) Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci USA 84: 109–112
Parson WW and Ke B (1982) Primary photochemical reactions. In: Photosynthesis I. Energy conversion by Plants and Bacteria (Govindjee ed), pp 331–385. New York: Academic Press
Provencher SW (1976) An eigenfunction expansion method for the analysis of exponential decay curves. J Chem Phys 64: 2772–2782
Satoh K, Fujii Y, Aoshima T and Tado T (1987) Immunological identification of the polypeptide bands in the SDS polyacrylamide gel electrophoresis of photosystem II preparations. FEBS Lett 216: 7–10
Schatz GH, Brock H and Holzwarth AR (1988) Kinetic and energetic model for the primary processes in photosystem II. Biophys J 54: 397–405
Seibert M, Picorel R, Rubin AB and Connolly JS (1988) Spectral, photophysical, and stability properties of isolated photosystem II reaction center. Plant Physiol 87: 303–306
Shipman LL, Norris JR and Katz JJ (1976) Quantum mechanical formalism for computation of the electronic spectral properties of chlorophyll aggregates. J Phys Chem 80: 877–882
Tetenkin VL, Gulyaev BA, Seibert M and Rubin AB (1989) Spectral Properties of Stabilized D1/D2/Cytochrome b-559 Photosystem II Reaction Center Complex. Effects of Triton X-100 and the Redox State of Pheophytin. Plant Physiol FEBS Lett. (in press)
Takahashi Y, Hansson O, Mathis P and Satoh K (1987) Primary radical pair in the photosystem II reaction centre. Biochim Biophys Acta 893: 49–59
van Gorkom HJ, Pulles MPJ and Wessels JSC (1975) Light-induced changes of absorbance and electron spin resonance in small photosystem II particles. Biochim Biophys Acta 408: 331–339
Wasielewski MR and Tiede DM (1986) Sub-picosecond measurements of primary electron transfer in Rhodopseudomonas viridis reaction centers using near-infrared excitation. FEBS Lett 204: 368–372
Wasielewski MR, Fenton JM and Govindjee (1987) The rate of formation of P700+ A0 − in photosystem I particles from spinach as measured by picosecond transient absorption spectroscopy. Photosynth Res 12: 181–190
Wasielewski MR, Johnson DG, Seibert M and Govindjee (1988) Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500 fs time resolution. Proceedings of the 10th International Congress on Photobiology, Abstracts, Kenes, Jerusalem, p. 82
Wasielewski MR, Johnson DG, Seibert M and Govindjee (1989) Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500 fs time resolution. Proc Natl Acad Sci USA 86: 524–528
Webber AN, Packman L, Chapman DJ, Barber J and Gray JC (1989) A fifth chloroplast encoded polypeptide is present in the photosystem II reaction centre complex. FEBS Lett 242: 259–262
Woodbury NW, Becker M, Middendorf D and Parson WW (1985) Picosecond kinetics of the initial photochemical electron transfer reaction in bacterial photosynthetic reaction centers. Biochemistry 24: 7516–7521
Yeates T-O, Komiya H, Rees DC, Allen JP and Feher G (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26: Membrane-protein interactions. Proc Natl Acad Sci USA 84: 6438–6442
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wasielewski, M.R., Johnson, D.G., Govindjee et al. Determination of the primary charge separation rate in Photosystem II reaction centers at 15 K. Photosynth Res 22, 89–99 (1989). https://doi.org/10.1007/BF00114769
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00114769