Abstract
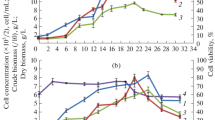
Cell suspensions ofMorinda citrifolia are able to produce large amounts of anthraquinones (AQ) when they are cultivated on a B5-medium containing 1 mg 1-1 naphtyl acetic acid (NAA); this production is inhibited by addition of 2,4-dichloro-phenoxyacetic acid (2,4-d). Also during cultivation on 1 mg 1-1 2,4-d AQ-production is absent.
It appeared that in the presence of NAA a kind of ‘AQ-production’ program is switched on: cell division rate is low as well as metabolic activity, while endogenous sugar levels are high. The same properties develop in the presence of auxins like indolyl-acetic acid and p-chloro-phenylacetic acid. With 2,4-d and related auxins (like p-chloro-phenoxyacetic acid) AQ production is absent and emphasis is laid on a developmental program characterized by high cell division rates, high metabolic activity and low endogenous sugar contents. Independent of the type of auxin applied, the cells grow as a suspension consisting of finely dispersed cells. The ‘AQ-producing differentiation program’ cannot be maintained during a consecutive series of subculturings: with increasing AQ-contents the viability of the cells and the cell division rate decrease.
The possible mechanisms of regulation of AQ-production by auxins are discussed as well as the advantages of the use of theMorinda model system in the study of the relation between growth, primary and secondary metabolism.
Similar content being viewed by others
Abbreviations
- AQ:
-
anthraquinones
- 2,4-d :
-
2,4-dichloro-phenoxylacetic acid
- DW:
-
dry weight
- EFW:
-
extractive free weight
- FW:
-
fresh weight
- IAA:
-
indolyl acetic acid
- NAA:
-
naphtyl acetic acid
- pCP:
-
p-chloro-phenylacetic acid
- pCPO:
-
p-chloro-phenoxy-acetic acid
References
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72: 248–254
De-Eknamkul W & Ellis BE (1985) Effects of auxins and cytokinins on growth and rosmarinic acid formation in cell suspension cultures ofAnchusa officinalis. Plant Cell Rep. 4: 50–53
Gamborg OL, Miller RA & Ojima V (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
Hagendoorn MJM, Traas TP, Boon JJ & Van der Plas LHW (1990) Orthovanadate induced lignin production, in batch and continuous cultures ofPetunia hybrida. J. Plant Physiol. 137: 72–80
Hagendoorn MJM, Van der Plas LHW & Segers GJ (1994a) Accumulation of anthraquinones inMorinda citrifolia cell suspensions. Plant Cell Tissue Org. Cult. 38: 227–234
Hagendoorn MJM, Wagner AM, Segers G, Van der Plas LHW, Oostdam A & Van Walraven HS (1994b) Cytoplasmic acidification and secondary metabolite production in different plant cell suspensions. A comparative study. Plant Physiol. 106: 723–730
Hoekstra FA, Crowe LM & Crowe JH (1989) Differential desiccation sensitivity of corn andPennisetum pollen linked to their sucrose content. Plant Cell Environ. 12: 83–91
Inoue K, Nayeshiro H, Inouye H & Zenk M (1981) Anthraquinones in cell suspension cultures ofMorinda citrifolia. Phytochemistry 20: 1693–1700
Mokrasch LC (1954) Analysis of hexose phosphate and sugar mixtures with the anthrone reagent. J. Biol. Chem. 208: 55–59
Sato F & Yamada Y (1984) High berberine-producing cultures ofCoptis japonica cells. Phytochemistry 23: 281–285
Ulbrich B, Wiesner W & Arens H (1985) Large-scale production of rosmarinic acid from plant cell cultures ofColeus blumei Benth. In: Neumann K-H, Barz W & Reinhard E (Eds) Primary and Secondary Metabolism of Plant Cell Cultures (pp 293–303). Springer-Verlag, Berlin, Heidelberg
Zenk MH, EI-Shagi H & Schulte U (1975) Anthraquinone production by cell suspension cultures ofMorinda citrifolia. Planta Med. Suppl. 79–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Plas, L.H.W., Eijkelboom, C. & Hagendoorn, M.J.M. Relation between primary and secondary metabolism in plant cell suspensions. Plant Cell Tiss Organ Cult 43, 111–116 (1995). https://doi.org/10.1007/BF00052164
Issue Date:
DOI: https://doi.org/10.1007/BF00052164