Abstract
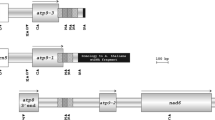
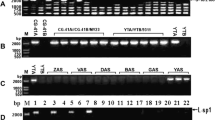
In sunflower plants carrying the PET1 cytoplasm male sterility (CMS) is associated with a new open reading frame (orfH522) in the 3′-flanking region of the atpA gene and an additional 16 kDa protein. Twenty-seven male-sterile cytoplasms of different origin were studied for the expression of the 16 kDa protein. In addition to the PET1 cytoplasm nine other male-sterile cytoplasms express the CMS-associated protein. These CMS sources originate from different interspecific crosses, from spontaneously occurring male-sterile plants in wild sunflower and from induced mutagenesis. Polyclonal antisera were raised against fusion proteins which contain 421 bp of the 3′-coding region of orfH522 to verify by immunological methods the identity of the protein in the other CMS cytoplasms. The anti-ORFH522 antiserum showed a positive reaction in the immunoblot with all CMS cytoplasms expressing the 16 kDa protein. Investigations of the mitochondrial DNA demonstrated that all ten CMS cytoplasms which express the 16 kDa protein have the same organization at the atpA locus. OrfH522 is located in the 3′-flanking region of the atpA gene. Transcript analyses using atpA and orfH522 as probes gave the same transcript pattern for the investigated CMS cytoplasms, just as for PET1. The MAX1 cytoplasm has an orfH522-related sequence but does not synthesize the 16 kDa protein. Using the sodium carbonate treatment the 16 kDa protein proved to be membrane-bound. Computer analyses predict that the hydrophobic N-terminal region of ORFH522 may form a transmembrane helix functioning as membrane anchor.
Similar content being viewed by others
References
Abad AR, Mehrtens BJ, Mackenzie SA: Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated protein in cytoplasmic male-sterile bean. Plant Cell 7: 271–285 (1995).
Bailey-Serres J, Hanson DK, Liddell AD, Leaver CJ: Nuclear-mitochondrial interactions in cytoplasmic male-sterile Sorghum. Theor Appl Genet 73: 252–260 (1986).
Bonner WM, Laskey RA: A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 46: 83–88 (1974).
Chou PY, Fasman DG: Empirical predicitions of protein conformation. Annu Rev Biochem 47: 251–276 (1978).
Christov M: New sources of male sterility and opportunities for their utilization in sunflower hybrid breeding. Helia 15: 41–48 (1992).
Cooper P, Newton KJ: Maize nuclear background regulates the synthesis of a 22 kDa polypeptide in Zea luxurians mitochondria. Proc Natl Acad Sci USA 86: 7423–7426 (1989).
Crouzillat D, de la Canal L, Perrault A, Ledoigt G, Vear F, Serieys H. Cytoplasmic male sterility in sunflower: comparison of molecular biology and genetic studies. Plant Mol Biol 16: 415–426 (1991).
Crouzillat D, de la Canal L, Vear F, Serieys H, Ledoigt G: Mitochondrial DNA RFLP and genetical studies of cytoplasmic male sterility in the sunflower (Helianthus annuus). Curr Genet 26: 146–152 (1994).
Dewey RE, Timothy DH, LevingsIII CS: A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci 84: 5374–5378 (1987).
Eisenberg D, Weiss RM, Terwillinger TC: The helical hydrophobic moment: a measure of the amphiplicity of a helix. Nature 299: 371–374 (1982).
Eisenberg D, Schwarz E, Komaromy M, Wall R: Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol 179: 125–142 (1984).
Firestone GL, Winguth SD: Immunoprecipitation of proteins. Meth Enzymol 182: 688–700 (1990).
Forde BG, Oliver RJC, Leaver CJ: Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci USA 75: 3841–3845 (1978).
Friedt W: Present state and future prospects of biotechnology in sunflower breeding. Field Crops Res 30: 425–442 (1992).
Fujiki Y, Hubbard AL, Fowler S, Lazarow PB: Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmatic reticulum. J Cell Biol 93: 97–102 (1982).
Garnier DJ, Osgurthorpe J, Robson B: Analysis of the accuracy and implications of simple methods for predictiting the secondary structure of globular proteins. J Mol Biol 120: 97–120 (1978).
Grelon M, Budar F, Bonhomme S, Pelletier G: Ogura cytoplasmic male-sterility CMS-associated orf138 is translated into a mitochondrial membrane polypeptide in male-sterile Brassica cybrids. Mol Gen Genet 243: 540–547 (1994).
Hack E, Lin C, Yang H, Horner HT: T-URF13 protein from mitochondria of Texas male-sterile maize (Zea mays L.). Plant Physiol 95: 861–870 (1991).
Hahn V, Friedt W: Molecular analysis of the cms-inducing MAX1 cytoplasm in sunflower. Theor Appl Genet 89: 379–385 (1994).
Håkansson G, Van der Mark F, Bonnett HT, Glimelius K: Variant mitochondrial protein and DNA patterns associated with cytoplasmic male-sterile lines of Nicotiana. Theor Appl Genet 76: 431–437 (1988).
Haveskes FWJ, Miller JF, Jan CC: Diversity among sources of cytoplasmic male sterility in sunflower (Helianthus annuus L.). Euphytica 55: 125–129 (1992).
Hiesel R, Schobel, Schuster W, Brennicke A. The cytochrome subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promotor sequences. EMBO J 6: 29–34 (1987).
Horn R, Köhler RK, Zetsche K: A mitochondrial 16 kDa protein is associated with cytoplasmic mlae sterility in sunflower. Plant Mol Biol 17: 29–36 (1991).
Köhler RK, Horn R, Lössl A, Zetsche K: Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol Gen Genet 227: 369–376 (1991).
Korth Kl, Kaspi CI, Siedow JN, Levings CSIII: URF13, a maize mitochondrial pore-forming protein, is oligomeric and has a mixed orientation in Escherichia coli plasma membranes. Proc Natl Acad Sci USA 88: 10865–10869 (1991).
Kyte J, Doolittle RF: A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105–132 (1982).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Laver HK, Reynolds SJ, Moneger F Leaver CJ: Mitochondrial genome organization and expression associated with cytoplasmic male sterility in sunflower (Helianthus annuus). Plant J 1: 185–193 (1991).
Leaver CJ, Hack E, Forde BG: Protein synthesis by isolated plant mitochondria. Meth Enzymol 97: 476–484 (1983).
Leclercq P: Une stérilité mâle chez le tournesol. Ann Amélior Plantes 19: 99–106 (1969).
Levin JM, Garnier J: Improvements in a secondary structure prediction method based on a search for local sequence homologies and its use as a model building tool. Biochim Biophys Acta 955: 283–295 (1988).
Miller JF: Update on inheritance of sunflower characteristics. In: Proceedings 13th International Sunflower Conference (Pisa, Italy), pp. 905–945 (1992).
Monéger F, Smart CJ, Leaver CJ: Nuclear restoration of cytoplasmic male sterility in sunflower is associated with tissue-specific regulation of a novel mitochondrial gene. EMBO J 13: 8–17 (1994).
Nivison HT, Hanson MR: Identification of a mitochondrial protein associated with cytoplasmic male sterility in Petunia. Plant Cell 1: 1121–1130 (1989).
Potz H, Tatlioglu T: Molecular analysis of cytoplasmic male sterility in chives (Allium schoenoprasum L.). Theor Appl Genet 87: 439–445 (1993).
Ptitsyn OB, Finkelstein AV: Theory of protein secondary structure and algorithm of its prediction. Biopolymers 22: 15–25 (1983).
Rieseberg LH, Van Fossen C, Adrias D, Carter RL: Cytoplasmic male sterility in sunflower: origin, inheritance, and frequency in natural populations. J Hered 85: 233–238 (1994).
Rogers SO, Bendich AJ: Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5: 69–76 (1985).
Rost B, Sander C: Prediction of protein structure at better than 70% accuracy. J Mol Biol 232: 584–599 (1993).
Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Siculella L, Palmer JD: Physical and gene organization of mitochondrial DNA in fertile and male-sterile sunflower. Nucl Acids Res 16: 3787–3799 (1988).
Spassova M, Christov M, Bohorova N, Petrov P, Dudov K, Atanassov A, Nijkamp HJ, Hille J: Molecular analysis of a new cytoplasmic male-sterile genotype in sunflower. FEBS Lett 297: 159–163 (1992).
Spassova M, Monéger F, Leaver CJ, Petrov P, Atanassov A, Nijkamp JJ, Hille J: Characterisation and expression of the mitochondrial genome of a new type of cytoplasmic male-sterile sunflower. Plant Mol Biol 26: 1819–1831 (1994).
Zetsche K, Horn R: Molecular analysis of cytoplasmic male sterility in sunflower. In: Brennicke A, Kück U (eds) Plant Mitochondria, pp. 419–422. Springer-Verlag, Weinheim/New York, NY (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Horn, R., Hustedt, J.E.G., Horstmeyer, A. et al. The CMS-associated 16 kDa protein encoded by orfH522 in the PET1 cytoplasm is also present in other male-sterile cytoplasms of sunflower. Plant Mol Biol 30, 523–538 (1996). https://doi.org/10.1007/BF00049329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00049329