Abstract
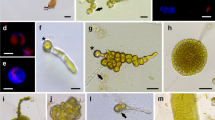
Viable protoplasts were isolated from apices of the agarophyte Gelidium robustum (Gardn.) Hollenb. & Abb. using a combination of commercial cell-wall degrading enzymes and extracellular wall-degrading enzymes isolated from a marine bacterium. The protoplasts were approximately 8–15 µm in diameter, liberated mainly from the surface cell layers and from cells at the distal ends of medullary filaments. The bacterial enzyme alone was not sufficient to liberate significant numbers of protoplasts. Maximum yield was 9 × 105 protoplasts/g tissue (wet wt.). Optimum osmolality occurred between 1750–1950 mOs kg−1; yield and viability were severely diminished at osmolalities less than 1350 mOs kg−1. Viability, as determined by flurorescein diacetate staining and Evans Blue exclusion 1 hr after removal from the enzyme solution, was approximately 80–95%. Roughly 80% of the cells did not show Calcofluor fluorescence, while 40% stained positively for the presence of sulfated polysaccharides. Cell wall regeneration was observed with inconsistent reproducibility, and no cell division was observed when the protoplasts were placed in culture medium.
Similar content being viewed by others
References
Albersheim, P. & A. G. Darvill, 1985. Oligosaccharins. Sci. Amer. 253: 58–64.
Araki, C., 1966. Some recent studies on the polysaccharides of agarophytes. Proc. int. Seaweed Symp. 5: 3–17.
Bjork, M., P. Ekman, A. Wallin & M. Pedersen, 1990. Effects of growth rate and other factors on protoplasts of four species of Gracilaria (Rhodophyta). Bot. mar. 33: 433–439.
Chen, L. C. M., 1989. Cell suspension cultures from Porphyra linearis (Rhodophyta), a multicellular marine red alga. J. appl. Phycol. 1: 153–159.
Cheney, D., E. Mar, N. Saga & J. van der Meer. 1986. Protoplast isolation and cell division in the agar-producing seaweed Gracilaria (Rhodophyta). J. Phycol. 22: 238–243.
Craigie, J. S. & Z. C. Wen. 1984. Effects of temperature and tissue age on gel strength and agar composition from Gracilaria tikvahiae (Rhodophyceae). Can. J. Bot. 62: 1665–1670.
Galbraith, D. 1981. Microfluorimetric quantitation of cellulose biosynthesis by plant protoplasts using Calcofluor White. Physiol. Pl. 53: 111–116.
Galbraith, D. 1989. Analysis of higher plants by flow cytometry and cell sorting. Int. Revue. Cytol. 116: 165–228.
Gross, W. 1990. Preparation of protoplasts from the carrageenophyte Gigartina corymbifera (Kutz.) J. Ag. (Rhodophyta). J. microb. Meth. 12: 217–223.
Larkin, P. J. 1976. Purification and viability determinations of plant protoplasts. Planta (Berl.) 128: 213–216.
Le Gall, Y., J. P. Braud & B. Kloareg, 1990. Protoplast production in Chondrus crispus gametophytes (Gigartinales, Rhodophyta). Pl. Cell Reports 8: 582–585.
Liu, Q. Y., L. C-M. Chen & A. R. A. Taylor, 1992. Ultrastructure of cell wall regeneration by isolated protoplasts of Palmaria palmata (Rhodophyta). Bot. mar. 35: 21–33.
Liu, X. W., C. Rochas & B. Kloareg, 1990. Callus culture of Pterocladia capillacea (Rhodophyta) and analysis of cell wall polysaccharides. J. appl. Phycol. 2: 297–303.
McCully, M. 1970. The histological localization of the structural polysaccharides in seaweeds. Ann. N.Y. Acad. Sci. 175: 702–711.
McHugh, D. J., 1991. Worldwide distribution of commercial resources of seaweeds including Gelidium. In J. A. Juanes, B. Santelices & J. L. McLachlan (eds), International Workshop on Gelidium. Developments in Hydrobiology 68. Kluwer Academic Publishers, Dordrecht: 19–30. Reprinted from Hydrobiologia 221.
Naganuma, T., D. A. Coury, M. Polne-Fuller, A. Gibor & K. Honkoshi, 1993. Characterization of agarolytic Microscilla isolates and their extracellular agarases. System appl. Microb. 16: in press.
Polne-Fuller, M. 1991. A novel technique for preparation of axenic cultures of Symbiodinium (Pyrrophyta) through selective digestion by amoebae. J. Phycol. 27: 552–554.
Polne-Fuller, M. & A. Gibor, 1984. Developmental studies in Porphyra. I. Blade differentiation in Porphyra perforata as expressed by morphology, enzymatic digestion, and protoplast regeneration. J. Phycol. 20: 609–616.
Provasoli, L., 1968. Media and prospects for the cultivation of marine algae. In A. Watanabe & A. Hattori (eds), Cultures and Collections of Algae. Proc. U.S.-Japan Conf. Hakone, Sept. 1966. Jap. Soc. Plant Physiol.: pp. 63–75.
Santelices, B., 1991. Production ecology of Gelidium. In J. A. Juanes, B. Santelices & J. L. McLachlan (eds), International Workshop on Gelidium. Developments in Hydrobiology 68. Kluwer Academic Publishers, Dordrecht: 31–44. Reprinted from Hydrobiologia 221.
Tait, M. I., A. M. Milne, D. Grant, J. A. Somers, J. Staples, W. F. Long, F. B. Williamson & S. B. Wilson. 1990. Porphyra cell cultures: isolation, growth and polysaccharide production. J. appl. Phycol. 2: 63–70.
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Professor Michael Neushul.
Rights and permissions
About this article
Cite this article
Coury, D.A., Naganuma, T., Polne-Fuller, M. et al. Protoplasts of Gelidium robustum (Rhodophyta). Hydrobiologia 260, 421–427 (1993). https://doi.org/10.1007/BF00049051
Issue Date:
DOI: https://doi.org/10.1007/BF00049051