Abstract
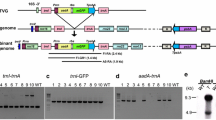
cDNA clones for pea plastocyanin were isolated from a pea leaf cDNA library screened with a 32P-labelled mixed oligonucleotide probe predicted from part of the N-terminal amino acid sequence of pea plastocyanin. The six cDNA clones isolated were found to be identical in the regions in which they overlapped. A Southern blot of restricted pea DNA probed with one of these cDNA clones showed the pea plastocyanin gene to exist as a single copy in the haploid genome. A pea genomic library in λEMBL3 screened with the same cDNA clone gave three positive plaques which contained identical 16 kbp Bam HI fragments. A single uninterrupted plastocyanin gene was located near the middle of the fragment and was characterised by DNA sequencing. The derived amino acid sequence indicates that the plastocyanin precursor consists of 168 amino acid residues including a presequence of 69 amino acid residues. The transcription initiation site was located by S1 nuclease mapping approximately 50 bp upstream of the translation initiation site. A sequence similar to a consensus light-responsive element found in a large number of phytochrome-dependent light-inducible genes is located just upstream of the TATA box. A cluster of direct repeats containing potential Z-DNA-forming elements occurs 600–750 bp upstream of the transcription initiation site.
Similar content being viewed by others
References
An G: A potential Z-DNA-forming sequence is an essential upstream element of a plant promoter. BioEssays 7: 211–214 (1987).
Bennett MD, Smith JB: Nuclear DNA amounts in angiosperms. Phil Trans Roy Soc Lond Ser B 274: 227–274 (1976).
Benton WD, Davis RW: Screening λgt11 recombinant clones by hybridisation to single plaques in situ. Science 196: 180–182 (1977).
Berk AJ, Sharp PA: Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease digested hybrids. Cell 12: 721–732 (1977).
Bonner WM, Laskey RA: A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 46: 83–88 (1974).
Boulter D, Haslett BG, Peacock D, Ramshaw JAM, Scawen MD: Chemistry, function and evolution of plastocyanin. In: Northcote DH (ed) International Review of Biochemistry 13, Plant Biochemistry II, pp. 1–40. University Park Press, Baltimore (1977).
Boulter D, Peacock D, Guise A, Gleaves JT, Estabrook G: Relationships between the partial amino acid sequences of plastocyanin from members of ten families of flowering plants. Phytochemistry 8: 603–608 (1979).
Cashmore A: Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding protein polypeptide. Proc Natl Acad Sci USA 81: 2960–2964 (1984).
Coruzzi G, Broglie R, Cashmore A, Chua N-H: Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem 258: 1399–1402 (1983).
Coruzzi G, Broglie R, Edwards C, Chua N-H: Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J 3: 1671–1679 (1984).
Domoney C, Casey R: Measurement of gene number for seed storage proteins in Pisum. Nucleic Acids Res 13: 687–699 (1985).
Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Fluhr R, Kuhlemeier C, Nagy F, Chua N-H: Organ-specific and light-induced expression of plant genes. Science 232: 1106–1112 (1986).
Frischauf A-M, Lehrach H, Poustika A, Murray N: Lambda replacement vectors carrying polylinker sequences. J Mol Biol 170: 827–842 (1983).
Grob U, Stuber K: Discrimination of phytochrome dependent light inducible from non-light inducible plant genes. Prediction of a common light-responsive element (LRE) in phytochrome dependent light inducible plant genes. Nucleic Acids Res 15: 9957–9973 (1987).
Grossman AR, Bartlett SG, Schmidt GW, Mullet JE, Chua N-H: Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. J Biol Chem 257: 1558–1563 (1982).
Gubler U, Hoffman BJ: A simple and very efficient method for generating cDNA libraries. Gene 25: 263–269 (1983).
Hageman J, Robinson C, Smeekens S, Weisbeek P: A thylakoid processing protease is required for complete maturation of the lumen protein plastocyanin. Nature 324: 567–569 (1986).
Hanahan D: Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166: 557–580 (1983).
Haslett BG, Cammack R: The development of plastocyanin in greening bean leaves. Biochem J 144: 567–572 (1974).
Howe CJ, Bowman CM, Dyer TA, Gray JC: Localisation of wheat chloroplast genes for the beta and epsilon subunits of ATP synthase. Mol Gen Genet 186: 525–530 (1982).
Jackson RJ, Hunt T: Preparation and use of nucleasetreated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol 96: 50–74 (1983).
Karlin-Neumann GA, Tobin EM: Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J 5: 9–13 (1986).
Kirwin PM, Elderfield PD, Robinson C: Transport of proteins into chloroplasts. Partial purification of a thylakoidal peptidase involved in plastocyanin biogenesis. J Biol Chem 262: 16386–16390 (1987).
Kozak M: Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res 9: 5233–5252 (1981).
Laemmi UK: Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685 (1970).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Munual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
Merchant S, Bogorad L: Regulation by copper of the expression of plastocyanin and cytochrome c-522 in Chlamydomonas reinhardtii. Mol Cell Biol 6: 462–469 (1986).
Messing J: New M13 vectors for cloning. Meth Enzymol 101: 20–78 (1983).
Morelli G, Nagy F, Fraley RT, Rogers SG, Chua N-H: A short conserved sequence is involved in the light-inducibility of a gene encoding ribulose 1,5-bisphosphate carboxylase small subunit of pea. Nature 315: 200–204 (1985).
Newman BJ, Gray JC: Characterisation of a full-length cDNA clone for pea ferredoxin-NADP+ reductase. Plant Mol Biol 10: 511–520 (1988).
Plesnicar M, Bendall DS: The plastocyanin content of chloroplasts from some higher plants estimated by a sensitive enzymic assay. Biochim Biophys Acta 216: 192–199 (1970).
Plesnicar M, Bendall DS: The photochemical activities and electron carriers of developing barley leaves. Biochem J 136: 803–812 (1973).
Robinson C, Ellis RJ: Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of imported precursor polypeptides. Eur J Biochem 142: 337–342 (1984).
Rother C, Jansen T, Tyagi A, Tittgen J, Herrmann RG: Plastocyanin is encoded by an uninterrupted nuclear gene in spinach. Curr Genet 11: 171–176 (1986).
Sanger F, Coulson AR, Barrel BG, Smith AJH, Roe BA: Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol 143: 161–178 (1980).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Smeekens S, Bauerle C, Hageman J, Keegstra K, Weisbeek P: The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell 46: 365–375 (1986).
Smeekens S, DeGroot M, VanBinsbergen J, Weisbeek P: Sequence of the precursor of the chloroplast thylakoid lumen protein, plastocyanin. Nature 317: 456–458 (1985).
Smeekens S, VanBinsbergen J, Weisbeek P: The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res 13: 3179–3194 (1985).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517 (1975).
Takabe T, Ishikawa H, Niwa S, Tanaka Y: Electron transfer reactions of chemically modified plastocyanin with P700 and cytochrome % MathType!MTEF!2!1!+-% feaafiart1ev1aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr% 4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq-Jc9% vqaqpepm0xbba9pwe9Q8fs0-yqaqpepae9pg0FirpepeKkFr0xfr-x% fr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaaccaGae8NKby% kaaa!37B5!\[f\]. Importance of local charges. J Biol Chem 96: 385–393 (1986).
Takabe T, Takabe T, Akazawa AT: Biosynthesis of P700-chlorophyll a protein, plastocyanin, and cytochrome b 6-% MathType!MTEF!2!1!+-% feaafiart1ev1aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr% 4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq-Jc9% vqaqpepm0xbba9pwe9Q8fs0-yqaqpepae9pg0FirpepeKkFr0xfr-x% fr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaaccaGae8NKby% kaaa!37B5!\[f\] complex. Plant Physiol 81: 60–66 (1986).
Thomas P: Hybridisation of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77: 5201–5205 (1980).
Thompson WF, Everett M, Polans NO, Jorgensen RA, Palmer JD: Phytochrome control of RNA levels in developing pea and mung-bean leaves. Planta 158: 487–500 (1983).
Tittgen J, Hermans J, Steppuhn J, Jansen T, Jansson C, Andersson B, Nechushtai R, Nelson N, Herrmann RG: Isolation of cDNA clones for fourteen nuclear-encoded thylakoid membrane proteins. Mol Gen Genet 204: 258–265 (1986).
VonHeijne G: Patterns of amino acids near signalsequence cleavage sites. Eur J Biochem 133: 17–21 (1983).
VonHeijne G: Signal sequences. The limits of variation. J Mol Biol 184: 99–105 (1985).
Vorst O, Oosterhoff-Teerstra R, Vankan P, Smeekens S, Weisbeek P: Plastocyanin of Arabidopsis thaliana: isolation and characterization of the gene and chloroplast import of the precursor protein. Gene 65: 59–69 (1988).
Waye MMY, Verhoeyen ME, Jones PT, Winter G: EcoK selection vectors for shotgun cloning into M13 and deletion mutagenesis. Nucleic Acids Res 13: 8561–8571 (1985).
Weaver RF, Weissmann C: Mapping of RNA by a modification of the Berk-Sharp procedure: the 5′ termini of the 15S β-globin mRNA precursor and mature 10S β-globin mRNA have identical map coordinates. Nucleic Acids Res 7: 1175–1193 (1979).
Wood PM: Interchangeable Cu and Fe proteins in algal photosynthesis. Eur J Biochem 87: 9–19 (1978).
Wood WB: Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol 16: 118–133 (1966).
Yanisch-Perron C, Veiera J, Messing J: Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33: 103–119 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Last, D.I., Gray, J.C. Plastocyanin is encoded by a single-copy gene in the pea haploid genome. Plant Mol Biol 12, 655–666 (1989). https://doi.org/10.1007/BF00044156
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00044156