Abstract
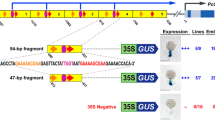
E4 gene transcription is controlled by ethylene during tomato fruit ripening. To define the ethylene-responsive promoter elements, we have tested the activity of mutations of the E4 promoter, and of chimeric genes in transient assay. Using a set of linker scan mutations of the region from -160 to -91, we determined that sequences located between -150 and -121 bp from the transcription start site are required for normal levels of ethylene-regulated transcription. However, E4 sequences from -193 to -40 were not able to confer ethylene-responsiveness to the minimal (-46) 35S promoter. The E4/E8 binding protein (E4/E8 BP) interacts with sequences in the 5′-flanking regions of both E4 and the coordinately regulated E8 gene, and its role in regulation of E4 transcription was investigated. The E4 binding site spans the E4 TATA box, and so mutations of this site were limited to those that did not disrupt the E4 TATA box. Mutations of this site which reduced affinity for the E4/E8 BP also resulted in reduced activity in transient assay, supporting a role for this element in normal regulation of the gene. Fusion of the 35S enhancer to E4 sequences from -85 to +65 did not result in an ethylene-responsive promoter, indicating that the E4/E8 BP-binding site is not sufficient for ethylene response. We conclude that at least twocis elements are required for ethylene-responsive transcription of the E4 gene during fruit ripening, one between -150 and -121 and the other between -40 and +65.
Similar content being viewed by others
References
Abeles FB, Morgan PW, Saltveit MEJr: Ethylene in Plant Biology. Academic Press, San Diego (1992).
Bird CR, Smith CJS, Ray JA, Moureau P, Bevan MW, Bird AS, Hughes S, Morris PC, Grierson D, Schuch W: The tomato polygalacturonase gene and ripening-specific expression in transgenic plants. Plant Mol Biol 11: 651–662 (1988).
Brady CJ: Fruit ripening. Annu Rev Plant Physiol 38: 155–178 (1987).
Cordes S, Deikman J, Margossian LJ, Fischer RL: Interaction of a developmentally regulated DNA-binding factor with sites flanking two different fruit-ripening genes from tomato. Plant Cell 1: 1025–1034 (1989).
Deikman J, Fischer RL: Interaction of a DNA binding factor with the 5′-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J 7: 3315–3320 (1988).
Deikman J, Kline R, Fischer RL: Organization of ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicon esculentum). Plant Physiol 100: 2013–2017 (1992).
DellaPenna D, Lincoln JE, Fischer RL, Bennett AB: Transcriptional analysis of polygalacturonase and other ripening associated genes in Rugters,rin, nor, andNr tomato fruit. Plant Physiol 90: 1372–1377 (1989).
Fang R-X, Nagy F, Sivasubramaniam S, Chua N-H: Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell 1: 141–150 (1989).
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR: Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59 (1989).
Itzhaki H, Maxson JM, Woodson WR: An ethylene-responsive enhancer elements is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc Natl Acad Sci USA 91: 8925–8929 (1994).
Jefferson RA: Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Kim JL, Nikolov DB, Burley SK: Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 365: 520–527 (1993).
Kunkel TA, Roberts JD, Zakour RA: Rapid and efficient site-specific mutagenesis without phenotypic selection. Meth Enzymol 154: 367–382 (1987).
Lincoln JE, Cordes S, Read E, Fischer RL: Regulation of gene expression by ethylene duringLycopersicon esculentum (tomato) fruit ripening. Proc Natl Acad Sci USA 84: 2793–2797 (1987).
Lincoln JE, Fischer RL: Diverse mechanisms for the regulation of ethylene-inducible gene expresion. Mol Gen Genet 212: 71–75 (1988).
Lincoln JE, Fischer RL: Regulation of gene expression by ethylene in wild-type andrin tomato (Lycopersicon esculentum) fruit. Plant Physiol 88: 370–374 (1988).
Manzara T, Tarchevskaya S, Narita JO: Optimization of luciferase activity in a tomato transient assay system. Plant Mol Biol Rep 12: 221–226 (1994).
Montgomery J, Goldman S, Deikman J, Margossian L, Fischer RL: Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc Natl Acad Sci USA 90: 5939–5943 (1993).
Nicholass FJ, Smith CJS, Schuch W, Bird CR, Grierson D: High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol Biol 28: 423–435 (1995).
Oeller PW, Min-Wong L, Taylor L, Pike DA, Theologis A: Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254: 437–439 (1991).
Ohme-Takagi M, Shinshi H: Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 (1995).
Ow DW, Wood KV, DeLuca M, deWet JR, Helinski DR, Howell SH: Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234: 856–859 (1986).
Pabo CO, Sauer RT: Transcription factors: Structural families and principles of DNA recognition. Annu Rev Biochem 61: 1053–1095 (1992).
Rahman MA, Nelson H, Weissbach H, Brot N: Cloning, sequencing, and expression of theEscherichia coli peptide methionine sulfoxide reductase gene. J Biol Chem 267: 15549–15551 (1992).
Rogers JC, Lanahan MB, Rogers SW: Thecis-acting gibberellin response complex in high-pI alpha-amylase gene promoters. Plant Physiol 105: 151–158 (1994).
Rogers JC, Rogers SW: Definition and functional implications of gibberellin and abscisic acidcis-acting hormone response complexes. Plant Cell 4: 1443–1451 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY (1989).
Sessa G, Meller Y, Fluhr R: A GCC element and a G-box motif participate in ethylene-induced expression of the PRB-1b gene. Plant Mol Biol 28: 145–153 (1995).
Shinshi H, Usami S, Ohme-Takagi M: Identification of an ethylene-responsive region in the promoter of a tobacco class I chitinase gene. Plant Mol Biol 27: 923–932 (1995).
Terzaghi WB, Cashmore AR: Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 46: 445–474 (1995).
Vogeli-Lange R, Frundt C, Hart CM, Nagy F, Meins JF: Developmental, hormonal, and pathogenesis-related regulation of the tobacco class I beta-1,3-glucanase B promoter. Plant Mol Biol 25: 299–311 (1994).
Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ: An ethylene-inducible component of signal transduction encoded byNever-ripe. Science 270: 1807–1809 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, R., Goldman, S., Coupe, S. et al. Ethylene control of E4 transcription during tomato fruit ripening involves two cooperativecis elements. Plant Mol Biol 31, 1117–1127 (1996). https://doi.org/10.1007/BF00040829
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040829