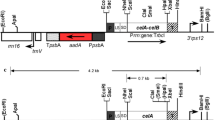
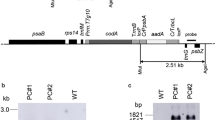
Abstract
A possible approach for altering alkaloid biosynthesis in plants is the expression of genes encoding key enzymes of a pathway such as lysine decarboxylase (ldc) in transgenic plants. Two strategies were followed here: one focused on expression of the gene in the cytoplasm, the other on subsequent targeting of the protein to the chloroplasts. Theldcgene fromHafnia alvei was therefore (a) placed under the control of the 1′ promoter of the bidirectional Tr promoter fromAgrobacterium tumefaciens Ti- plasmid, and (b) cloned behind therbcS promoter from potato fused to the coding region of therbcS transit peptide. Bothldc constructs, introduced intoNicotiana tabacum with the aid ofA. tumefaciens, were integrated into the plant genome and transcribed as shown by Southern and northern hybridization. However, LDC activity was only detectable in plants expressing mRNA under the control of therbcS promoter directing the LDC fusion protein into chloroplasts with the aid of the transit peptide domain. In plants expressing the processed bacterial enzyme cadaverine levels increased from nearly zero to 0.3–1% of dry mass.
Similar content being viewed by others
References
Arnon DJ: Copper enzymes in isolated chloroplasts. Polyphenoloxidase inBeta vulgaris. Plant Physiol 24: 1–15 (1949).
Beier H, Fecker LF, Berlin J: Lysinedecarboxylase fromHafnia alvei: Purification, molecular data and preparation of polyclonal antibodies. Z Naturforsch 42c: 1307–1312 (1987).
Berlin J, Fecker LF, Mollenschott C, Sator C, Strack D, Witte L, Wray V: Isoflavonoid formation in transformed and non-transformed suspension and hairy root cultures ofLupinus polyphyllus andL. hartwegii. Z Naturforsch 46c (in press).
Bolivar F, Rodriguez RL, Greene PJ, Betlach ML, Heyneker HL, Boyer HW, Crosa JH, Falkow S: Construction and characterization of new cloning vehicles II. a multipurpose cloning system. Gene 2: 95–113 (1977).
Cline K, Andrews J, Mersey B, Newcomb EH, Keegstra K: Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci USA 78: 3595–3599 (1981).
Dellaporta SL, Wood J, Hicks JB: A plant DNA minipreparation: Version II. Plant Mol Biol Rep 1: 19–21 (1983).
Depicker A, De Wilde M, De Vos G, De Vos R, Van Montagu M, Schell J: Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid 3: 193–211 (1980).
Ditta G, Stanfield S, Corbin D, Helinski DR: Broad host range DNA cloning system from gram-negative bacteria: Construction of a gene bank ofRhizobium meliloti. Proc Natl Acad Sci USA 77: 7347–7351 (1980).
Fecker LF, Beier H, Berlin J: Cloning and characterization of a lysine decarboxylase gene fromHafnia alvei. Mol Gen Genet 203: 177–184 (1986).
Fritz CC, Herget T, Wolter FP, Schell J, Schreier PH: Reduced steady state ofrbcS mRNA in plants kept in the dark are due to differential degradation. Proc Natl Acad Sci USA (in press).
Hain R, Stabel P, Czernilofsky AP, Steinbiss HH, Herrera-Estrella L, Schell J: Uptake, integration, expression and genetic transmission of a selectable chimeric gene by plant protoplasts. Mol Gen Genet 199: 161–168 (1985).
Hanahan D: Study on the transformation ofEscherichia coli with plasmids. J Mol Biol 166: 557–580 (1983).
Hamill JD, Robins RJ, Parr AJ, Evans DM, Furze JM, Rhodes MJC: Overexpressing a yeast ornithine decar-boxylase gene in transgenic roots ofNicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol Biol 15: 27–38 (1990).
Hartmann T: Quinolizidines and pyrrolizidines. In: Constabel F, Vasil IK (eds) Cell Culture and Somatic Cell Genetics of Plants, vol. 5; Phytochemicals in Plant Cell Cultures pp. 277–288. Academic Press, San Diego (1988).
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA: A linary plant vector strategy based on separation of virand T-region of theAgrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180 (1983).
Horsch RB, Fry J, Hoffmann N, Niedermeyer J, Rogers SG, Fraley RT: Leaf disc transformation. In: Gelvin SB, Schilperoort RA (eds) Plant Molecular Biology Manual, A5: 1–9 Kluwer Academic Publishers, Dordrecht (1988).
Johnson DA, Gautsch JW, Sportsman JR, Elder JH: Improved technique utilizing non fat dry milk for proteins and nucleic acids transferred to nitrocellulose. Gene Anal Techn 3: 1–8 (1984).
Krieg PA, Melton DA:In vitro RNA-synthesis with SP6 RNA-polymerase. Meth Enzymol 155: 397–415 (1987).
Kyhse-Andersen J: Electrolabelling of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Meth 10: 203–209 (1984).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685 (1970).
Landsmann J, Llewellyn D, Dennis ES, Peacock WJ: Organ regulated expression of theParasponia andersonii hemoglobine gene in transgenic tobacco plants. Mol Gen Genet 214: 68–73 (1988).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
Marton L, Wullems GJ, Molendijk L, Schilperoort RA:In vitro transformation of cultured cells fromNicotiana tabacum byAgrobacterium tumefaciens. Nature 277: 129–131 (1979).
Medgyesy P, Menzel L, Maliga P: The use of cytoplasmic streptomycin resistance: chloroplast transfer fromNicotiana tabacum intoNicotiana sylvestris and isolation of their somatic hybrids. Mol Gen Genet 179: 693–698 (1980).
Murashige T, Skoog F: A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Ngo TT, Brillhart KL, Davis RH, Wong RC, Bovaird JH, Digangi JJ, Ristow JL, Marsh JL, Phan APH, Lehnhoff HM: Spectrophotometric assay for ornithine decarboxylase. Anal Biochem 160: 290–293 (1987).
Odell JT, Caimi PG, Yadav NS, Mauvais CJ: Comparison of increased expression of wild-type and herbicide-resistant acetolactate synthase genes in transgenic plants, and indication of posttranscriptional limitation on enzyme activity. Plant Physiol 94: 1647–1654 (1990).
Read SM, Northcote DH: Minimizing of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem 116: 53–64 (1981).
Redmond JW, Tseng A: High pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. J Chromatogr 170: 479–481 (1979).
Reiss B, Sprengel R, Will H, Schaller H: A new sensitive method for qualitative assay of neomycin phosphototransferase in crude cell extracts. Gene 30: 211–218 (1984).
Reiss B, Wasmann CC, Schell J, Bohnert HJ: Effect of mutations on the binding and translocation functions of a chloroplast transit peptide. Proc Natl Acad Sci USA 86: 886–890 (1989).
Rigby PWJ, Dieckmann M, Rhodes C, Berg P: Labelling deoxyribonuleic acid to high specific activityin vitro by nick translation with DNA polymerase I. J Mol Biol 113: 237–251 (1977).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY (1989).
Schauder B, Blöcker H, Frank R, McCarthy JEG: Inducible expression vectors incorporating theEscherichia coli atpE translation initiation region. Gene 52: 279–283 (1987).
Schreier PH, Seftor EA, Schell J, Bohnert HJ: The use of nuclear encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J 4: 25–32 (1985).
Shillito RD, Paszkowski J, Potrykus I: Agarose plating and a bead type culture technique enable and stimulate development of protoplast-derived colonies in a number of plant species. Plant Cell Rep 2: 244–247 (1983).
Songstad DD, De Luca V, Brisson N, Kurz WGW, Nessler CL: High levels of tryptamine accumulation in transgenic tobacco expressing tryptophan decarboxylase. Plant Physiol 94: 1410–1413 (1990).
Teeri TH, Lehväslaiho H, Franck M, Uotila J, Heino P, Palva ET, Van Montagu M, Herrera-Estrella L: Gene fusions to lacZ reveal new expression patterns of chimeric genes in transgenic plants. EMBO J 8: 343–350 (1989).
Velten J, Schell J: Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucl Acids Res 13: 6981–6998 (1985).
Verwoerd TC, Dekker BMM, Hoekema A: A small scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362 (1989).
Wallsgrove RM, Mazelis M: The enzymology of lysine biosynthesis in higher plants. FEBS Lett 116: 189–192 (1980).
Wasmann CC, Reiss B, Bartlett SG, Bohnert HJ: The importance of the transit peptide and the transported protein for protein import into chloroplasts. Mol Gen Genet 205: 446–453 (1986).
Wink M, Hartmann T: Cadaverine-pyruvate transamination: The principal step of enzymatic quinolizidine alkaloid biosynthesis inLupinus polyphyllus cell suspension cultures. FEBS Lett 101: 343–346 (1979).
Wink M, Hartmann T: Sites of enzymatic synthesis of quinolizidine alkaloids and their accumulation inLupinus polyphyllus. Z Pflanzenphysiol 102: 337–344 (1981).
Wolter FP, Fritz CC, Willmitzer L, Schell J, Schreier PH: rbcS genes inSolanum tuberosum: Conservation of transit peptide and exon shuffling during evolution. Proc Natl Acad Sci USA 85: 846–850 (1988).
Zambryski P, Joos H, Genetello C, Leemans J, Van Montagu M, Schell J: Ti-plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2: 2143–2150 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herminghaus, S., Schreier, P.H., McCarthy, J.E.G. et al. Expression of a bacterial lysine decarboxylase gene and transport of the protein into chloroplasts of transgenic tobacco. Plant Mol Biol 17, 475–486 (1991). https://doi.org/10.1007/BF00040641
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040641