Abstract
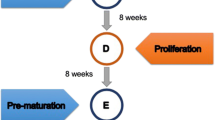
Starting at 8 weeks and continuing until 23 weeks (nut drop) after anthesis,1 m2 explants from cotyledons of immature seeds were extracted from Juglans nigra fruits. Explants were placed on Woody Plant Medium with 1 g l-1 casein hydrolysate and 30 g l-1 sucrose. The explants remained in light for 4 weeks on primary media containing a 3×3 factorial of 0.05, 0.5, or 5.0 μM thidiazuron (TDZ) and 0.1, 1.0, or 10.0 μM 2,4-d. Explants were transferred to a secondary medium containing no plant growth regulators and incubated in darkness for 11 weeks. The greatest number of somatic embryos was produced 8, 10, and 12 weeks after anthesis from explants on media with 0.5 or 5.0 μM TDZ and 0.1 or 1.0 μM 2,4-d. Explants produced the greatest callus volume and dry weight 10, 12, and 14 weeks after anthesis. Throughout the study, callus generally increased with increasing concentrations of both TDZ and 2,4-d.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- captan:
-
3a,4,7,7a-tetrahydro-2-[(trichloromethyl)thio]-1H-isoindole-1,3(2H)-dione
- 2,4-d :
-
2,4-dichlorophenoxyacetic acid
- IBA:
-
indolebutyric acid
- Physan:
-
n-alkyl- dimethyl-benzyl ammonium chlorides and n-alkyl-dimethyl-ethylbenzyl ammonium chlorides
- TDZ-thidiazuron:
-
N-phenyl-N′-1,2,3-thiadiazol-5-ylurea
References
Beineke, WF (1989) Selection and evaluation for better varieties. In: Phelps, JE (Ed) The Continuing Quest for Quality: Proceedings of the 4th Black Walnut Symposium; 1989 July 30-August 2; Carbondale, IL (pp 145–152). Walnut Council, Indianapolis, IN
Carpenter, BS (1975) Rooting black walnut cuttings with ethephon. Tree Planter's Notes 26(3): 3, 29
Cornu, D (1988) Somatic embryogenesis in tissue cultures of walnut (Juglans nigra, J. major and hybrids J. nigra * J. regia). In: Ahuja, MR (Ed) Somatic Cell Genetics of Woody Plants. (pp 45–49). Kluwer Academic Publishers, Dordrecht, The Netherlands
Cornu, D (1989) Walnut somatic embryogenesis: physiological and histological aspects. In: Dreyer, E, Aussenac, G, Bonnet-Masimbert, M, Dizengremel, P, Favre, JM, Garrec, JP, LeTacon, F & Martin, F (Eds) Ann. Sci. For. 46 Suppl. Forest Tree Physiology. (pp 133s-135s) Elsevier, Paris, France
Driver, JA & Kuniyuki, AH (1984) In vitro propagation of paradox walnut rootstock. HortScience 19: 507–509
Heile-Sudholt, CH, Huetteman, CA, Preece, JE, VanSambeek, JW & Gaffney, GR (1986) In vitro embryonic axis and seedling shoot tip culture of Juglans nigra L. Plant Cell Tiss. Org. Cult. 6: 189–197
Jay-Allemand, C, DePons, V, Doumas, P, Capelli, P, Sossountzov L & Cornu, D (1991) Formation de racines in vitro à partir de cotylédons de noix (Juglans sp.): un modèle d'étude de la rhozogénèse chez les espèces ligneuses. C.R. Acad. Sci. Paris Série III 312: 369–375
Jensen, WA (1962) Botanical Histochemistry, Freeman, San Francisco (p. 408)
Labavitch JM & Polito VS (1985) Fruit growth and development. In: Ramos DE (Ed) Walnut Orchard Management (pp 90–94). Univ. of CA-Davis Leaflet 21410
Liberty Hyde Bailey Hortorium (1976) Hortus Third: a Concise Dictionary of Plants Cultivated in the United States and Canada. 3rd edition Macmillan, New York
Lloyd, G & McCown, B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Intl. Plant Prop. Soc. 30: 421–427
Lockley, GC (1979) The effect of several concentrations of ethephon on breaking bud dormancy in black walnut. Tree Planter's Notes 30(4): 12–15
McGranahan, GH, Leslie, CA & Driver, JA (1988a) In vitro propagation of mature persian walnut cultivars. HortScience 23: 220
McGranahan, GH, Leslie, CA, Uratsu, SL, Martin, LA & Dandekar, AM (1988b) Agrobacterium-mediated transformation of walnut somatic embryos and regeneration of transgenic plants. Bio/Technology 6: 800–804
McGranahan, GH, Leslie, CA, Uratsu, SL & Dandekar, AM (1990) Improved efficiency of the walnut somatic embryo gene transfer system. Plant Cell Rep. 8: 512–516
Merkle, SA, Wetzstein, HY & Sommer, HE (1987) Somatic embryogenesis in tissue cultures of pecan. HortScience 22: 128–130
Preece, JE, VanSambeek, JW, Huetteman, CA & Gaffney, GR (1989) Biotechnology: In vitro studies with walnut (Juglans) species. In: Phelps, JE (Ed) The Continuing Quest for Quality: Proceedings of the 4th Black Walnut Symposium; 1989 July 30-August 2; Carbondale, IL (pp 159–180). Walnut Council, Indianapolis, IN
Reinert, J (1973) Aspects of organization-organogenesis and embryogenesis. In: Street, HE (Ed) Plant Tissue and Cell Culture (pp 338–355). Blackwell Scientific Publications, Oxford
Rink, G (1989) Recent advances in walnut tree improvement. In: Phelps, JE (Ed) The Continuing Quest for Quality: Proceedings of the 4th Black Walnut Symposium; 1989 Jyly 30-August 2; Carbondale, IL (pp 140–152). Walnut Council, Indianapolis, IN
Rodríguez, R (1982) Callus initiation and root formation from in vitro culture of walnut cotyledons. HortScience 17: 195–196
Sesco, JA (1989) Quality black walnut-through productive research, technology transfer, and cooperation. In: Phelps, JE (Ed) The Continuing Quest for Quality: Proceedings of the 4th Black Walnut Symposium; 1989 July 30-August 2; Carbondale, IL (pp 1–8). Walnut Council, Indianapolis, IN
Shreve LW (1974) Propagation of walnut, chestnut, and pecan by rooted cuttings. Proc. 8th Central States Forest Tree Improvement Conference, Oct. 11–13, 1972. Columbia MO
SAS (Statistical Analysis Systems) Institute (1982) SAS Users Guide. SAS Institute, Inc., Carey, NC
Steel RGD & Torrie JH (1980) Principles and Procedures of Statistics. A Biometrical Approach. 2nd Ed. McGraw-Hill
Tulecke, W & McGranahan, G (1985) Somatic embryogenesis and plant regeneration from cotyledons of walnut, Juglans regia L. Plant Sci. 40: 57–63
Van Sambeek JW & Rink G (1982) Physiology and silviculture of black walnut for combined timber and nut production. In: 72nd Annu Rep. Northern Nut Growers Assoc. pp 100–107
Young, PM, Hutchins, AS & Canfield, ML (1984) Use of antibiotics to control bacteria in shoot cultures of woody plants. Plant Sci. Lett. 34: 203–209
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neuman, M.C., Preece, J.E., Van Sambeek, J.W. et al. Somatic embryogenesis and callus production from cotyledon explants of Eastern black walnut. Plant Cell Tiss Organ Cult 32, 9–18 (1993). https://doi.org/10.1007/BF00040110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040110