Abstract
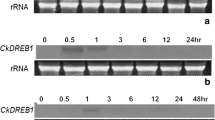
Exposure of seedlings of a chilling-sensitive variety of rice (Oryza sativa L. cv. Wasetoittu) to water stress (0.5 M mannitol, 30 min) at room temperature induced a degree of chilling resistance. No such resistance was induced by exogenous abscisic acid (ABA) application (10 μM, 60 min). Upon short-term water stress, new transcripts were expressed in both seedlings and suspension-cultured cells. We suggest that the genes induced by short-term water stress, and not those induced by ABA, are related to acquired chilling resistance in this chilling-sensitive rice variety. A total of nine different cDNA clones, specifically induced by short-term water stress, were isolated by differential hybridization and partial sequencing. Northern hybridization analysis using RNAs from the seedlings subjected to chilling after water stress treatment reveal three distinct groups of above mentioned nine cDNA clones: wsi (water stress-induced) 18, 76, and 724, representative of genes whose expression increases, decreases, and remains almost fixed during chilling, respectively. The nucleotide and deduced amino acid sequences of the three representative clones were determined. Characteristic features of wsi 18 are the presence of one set of amino acid sequence repeats, a conserved amino acid sequence common to LEA-group genes in the N-terminal region, and an alanine- and lysine-rich tract in the C-terminal region.
Similar content being viewed by others
References
Aguan K, Kusano T, Suzuki N, Kitagawa Y: An improved method of the construction of high efficency cDNA library in plasmid or lambda vector. Nucl Acids Res 18: 1071 (1990).
Almoguera C, Jordano J: Developmental and environmental concurrent expression of sunflower dry-seed-stored low-molecular-weight heat-shock protein and Lea mRNAs. Plant Mol Biol 19: 781–792 (1992).
Anderberg RJ, Walker-Simmons MK: Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinase. Proc Natl Acad Sci USA 89: 10183–10187 (1992).
Baker J, Steele C, DureIII L: Sequence and characterization of 6 lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291 (1988).
Bartels D, Hanke C, Schneider K, Michel D, Salamini F: A desiccation-related Elip-like gene from the resurrection plant Craterostigma plantagineum is regulated by light and ABA. EMBO J 11: 2771–2778 (1992).
Binh LT, Oono K: Molecular cloning and characterization of genes related to chilling tolerance in rice. Plant Physiol 99: 1146–1150 (1992).
Cattivelli L, Bartels D: Molecular cloning and characterization of cold-regulated genes in barley. Plant Physiol 93: 1504–1510 (1990).
Cavener DR, Ray S: Eukaryotic start and stop translation sites. Nucl Acids Res 19: 3185–3192 (1991).
Chen THH, Gusta LV: Abscisic acid-induced freezing resistance in cultured plant cells. Plant Physiol 73: 71–75 (1983).
Cohen A, Bray EA: Nucleotide sequence of an ABA-induced tomato gene that is expressed in wilted vegetative organs and developing seeds. Plant Mol Biol 18: 411–413 (1992).
Close TJ, Kortt AA, Chandler PM: A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol 13: 95–108 (1989).
Cox W: Interrelations between environmental factors and freezing resistance of cabbage leaves. Plant Physiol 57: 553–555 (1976).
Curry J, Morris CF, Walker-Simmons MK: Sequence analysis of a cDNA encoding a Group 3 LEA RNA inducible by ABA or dehydration stress in wheat. Plant Mol Biol 16: 1073–1076 (1991).
Drewe JA, Verma S, Frech G, Joho RH: Distinct spatial and temporal expression patterns of K+ channel mRNA from different subfamilies. J Neurosci 12: 538–548 (1992).
Dunn MA, Hughes MA, Zhang L, Pearce RS, Quigley AS, Jack PL: Nucleotide sequence and molecular analysis of the low temperature induced cereal gene, BLT4. Mol Gen Genet 229: 389–394 (1991).
Dunn MA, Hughes MA, Pearce RS, Jack PL: Molecular characterization of a barley gene induced by cold treatment. J Exp Bot 41: 1405–1413 (1990).
Dure LIII, Crouch M, Harada J, Ho T-HD, Mundy J, Quatrano R, Thomas T, Sung ZR: Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12: 475–486 (1989).
Fliegel L, Burns K, Macleannan DH, Reithmeier RAF, Michalak M: Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 264: 21522–21528 (1989).
Gilmour SJ, Artus NN, Thomashow MF: cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol 18: 13–21 (1992).
Gilmour SJ, Hajela RK, Thomashow MF: Cold acclimation in Arabidopsis thaliana. Plant Physiol 87: 745–750 (1988).
Godoy JA, Pardo JM, Pintor-Toro JA: A tomato cDNA inducible by salt stress and abscisic acid: nucleotide sequence and expression pattern. Plant Mol Biol 15: 695–705 (1990).
Gomez J, Sanchez-Martinez D, Stiefel V, Rigau J, Puigdomenech P, Pages M: A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 334: 262–264 (1988).
Guerrero FD, Jones JT, Mullet JE: Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol 15: 11–26 (1990).
Guy CL: Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41: 187–223 (1990).
Guy CL, Niemi KJ, Brambl R: Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci USA 81: 3673–3677 (1985).
Hahn M, Walbot V: Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol 91: 930–938 (1989).
Harada JJ, DeLisle AJ, Baden CS, Crouch ML: Unusual sequence of an abscisic acid-inducible mRNA which accumulates late in Brassica napus seed development. Plant Mol Biol 12: 395–401 (1989).
Hong B, Uknes SJ, Ho T-HD: Cloning and characterization of a cDNA encoding a mRNA rapidly-induced by ABA in barley aleurone layers. Plant Mol Biol 11: 495–506 (1988).
Joshi CP, King SW, Nguyen HT: Molecular cloning and characterization of a cDNA encoding a water stress protein (WSP23) from wheat roots. Plant Sci 86: 71–82 (1992).
King SW, Joshi CP, Nguyen HT: DNA sequence of an ABA-response gene (rab15) from water-stressed wheat roots. Plant Mol Biol 18: 119–121 (1992).
Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, Slaughter C, Reed RR, Gilman AG: Adenylyl cyclase amino acid sequence: possible channel-or transporter-like structure. Science 244: 1558–1564 (1989).
Koga-Ban Y, Abe M, Kitagawa Y: Alteration in gene expression during cold treatment of rice plant. Plant Cell Physiol 32: 901–905 (1991).
Kurkela S, Borg-Franck M: Structure and expression of Kin-2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol Biol 19: 689–692 (1992).
Kurkela S, Franck M: Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol Biol 15: 137–144 (1990).
Kusano T, Aguan K, Abe M, Sugawara K: Nucleotide sequence of a rice rab 16 homologue gene. Plant Mol Biol 18: 127–129 (1992).
Luo M, Lin L, Hill R, Mohapatra SS: Primary structure of an environmental and abscisic acid-inducible alfalfa protein. Plant Mol Biol 17: 1267–1269 (1991).
Luo M, Liu J-H, Mohapatra S, Hill RD, Mohapatra SS: Characterization of a gene family encoding abscisic acid- and environmental stress-inducible proteins of alfalfa. J Biol Chem 267: 15367–15374 (1992).
Masuda J, Kudo-shiratori A, Inoue M: Callus formation and plant regeneration from rice protoplasts purified by density gradient centrifugation. Plant Sci 62: 237–246 (1989).
Mdo V, Gomes SL: Cloning and structural analysis of the gene for the regulatory subunit of cAMP-dependent protein kinase in Blastocladiella emersonii. J Biol Chem 267: 17201–17207 (1992).
Monroy AF, Castonguay Y, Laberge S, Sarhan F, Vezina LP, Dhindsa RS: A new cold-induced alfalfa gene is associated with enhanced hardening at subzero temperature. Plant Physiol 102: 873–879 (1993).
Mundy J, Chua N-H: Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J 7: 2279–2286 (1988).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 (1962).
Neven LG, Haskell DW, Hofig A, Li Q-B, Guy CL: Characterization of a spinach gene responsive to low temperature and water stress. Plant Mol Biol 21: 291–305 (1993).
Nordin K, Vahala T, Palva ET: Differential expression of two related, low-temperature-induced genes in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 21: 641–653 (1993).
Ouellet F, Houde M, Sarhan F: Purification, characterization and cDNA cloning of the 200-kDa protein induced by cold acclimation in wheat. Plant Cell Physiol 34: 59–65 (1993).
Piatkowski D, Schneider K, Salamini F, Bartels D: Characterization of five abscisic acid-responsive cDNA clones isolated from the desiccation-tolerant plant Crateostigma plantagineum and their relation ship to other water-stress genes. Plant Physiol 94: 1682–1688 (1990).
Pla M, Gomez J, Goday A, Pages M: Regulation of the abscisic acid-responsive gene rab28 in maize viviparous mutants. Mol Gen Genet 230: 394–400 (1991).
Rikin A, Atsmon D, Gilter L: Chilling injury in cotton (Gossypium hirsutum L.): prevention by abscisic acid. Plant Cell Physiol 20: 1537–2546 (1979).
Roberts E, Kolattukudy PE: Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet 217: 223–232 (1989).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Shinozaki K, Mundy J, Chua N-H: Four tightly linked rab genes are differentially expressed in rice. Plant Mol Biol 14: 29–39 (1989).
Siminovitch D, Cloutier Y: Twenty-four-hours induction of freezing and drought tolerance in plumules of winter rye seedlings by desiccation stress at room temperature and in the dark. Plant Physiol 69: 250–255 (1982).
Tajima K, Amemiya A, Kabaki N: Physiological study of growth inhibition in rice plant as affected by low temperature II physiological mechanism and varietal difference of chilling injury in rice plant. Bull Natl Inst Agr Sci (Japan) D34: 69–111 (1983).
Thomashow MF: Molecular genetics of cold acclimation in higher plants. Adv Genet 28: 99–131 (1990).
Unkles SE, Campbell EI, deRuiter-Jacobs YMJT, Broekhuijsen M, Marco JA, Carrez D, Contreas R, van den Hondel CAMJJ, Kinghorn JR: The development of a homologous transformation system for Aspergillus oryzae based on the nitrate assimilation pathway: A convenient and general selection system for filamentous fungal fransformation. Mol Gen Genet 218: 99–104 (1989).
Vilardell J, Goday AJ, Freire MA, Torrent M, Martinez MC, Torne JM, Pages M: Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol 14: 423–432 (1990).
Walker-Sommons M: ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol 84: 61–66 (1987).
Weiler EW: Radioimmunoassay for the determination of free and conjugated abscisic acid. Planta 144: 255–263 (1979).
Yamaguchi-Shinozaki K, Shinozaki K: Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: Sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol 33: 217–224 (1992).
Yamaguchi-Shinozaki K, Shinozaki K: Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236: 331–340 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahashi, R., Joshee, N. & Kitagawa, Y. Induction of chilling resistance by water stress, and cDNA sequence analysis and expression of water stress-regulated genes in rice. Plant Mol Biol 26, 339–352 (1994). https://doi.org/10.1007/BF00039544
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039544