Abstract
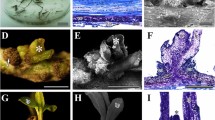
Adventitious shoot primordia were formed on horseradish hairy root cultured in dark. Plantlet formation frequency from the primordia was higher than that from root fragments. Culture for 26 days provided the adventitious shoot primordia, which had the highest potential for plantlet formation (53% explants at 40 days). Benzyladenine supplementation in the dark caused primordium enlargement, but did not increase the number of primordia formed. After adventitious shoot primordia were encapsulated with calcium alginate, kinetin supplementation (2.0–4.0 μM) increased the shoot formation frequency (65–80% explants at 20 days) in the light, but also promoted the undesirable formattion of multiple shoots. Supplementation with naphthaleneacetic acid (0.27–5.4 μM) in the calcium alginate beads in light enhanced the root emergence from primordia without inhibition of plantlet formation when the encapsulated beads were put on the agar-medium without naphthaleneacetic acid.
Similar content being viewed by others
References
Kato Y, Uozumi N, Kimura T, Honda H & Kobayashi T (1991) Enhancement of peroxidase production and excretion from horseradish hairy roots by light, NaCl, and peroxidase-adsorption in situ. Plant Tiss. Cult. Lett. 8: 158–265
Kilby NJ & Hunter CS (1990) Repeated harvest of vacuole-located secondary product from in vitro grown plant cells using 1.02 MHz ultrasound. Appl. Microbiol. Biotechnol. 33: 448–451
Kitto SL & Janick J (1985) Production of synthetic seeds by encapsulating asexual embryos of carrot. J. Amer. Soc. Hort. Sci. 110: 277–282
Kondo O, Honda H, Taya M & Kobayashi T (1989) Comparison of growth properties of carrot hairy root in various bioreactors. Appl. Microbiol. Biotechnol. 33: 291–294
Murashige T (1978) Plant growth substances in commercial uses of tissue culture. In: Thorpe TA (Ed) Frontiers of Plant Tissue Culture (pp 15–26). Internation Association of Plant Tissue Culture. Calgary
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Noda T, Tanaka N, Mano Y, Nabeshima S, Ohkawa H & Matsui C (1987) Regeneration of horseradish hairy roots incited byAgrobacterium rhizogenes infection. Plant Cell Rep. 6: 283–286
Ooms G, Karp A, Burrell MM, Twell D & Roberts J (1985) Genetic modification of potato development using Ri T-DNA. Theor. Appl. Genet. 70: 440–446
Pierik RLM (1987) In Vitro Culture of Higher Plants. Martinus Nijhoff Publishers, Dordrecht
Phelep M, Petit A, Martin L, Duhoux E & Tempé J (1991) Transformation and regeneration of a nitrogen-fixing tree,Allocasuarina verticillata Lam. Bio/Technology 9: 461–466
Redenbaugh K, Paasch BD, Nichol JW, Kossler ME, Viss PR & Walker K (1986) Somatic seeds: Encapsulation of asexual plant embryos. Bio/Technology 4: 797–801
Shimomura K, Sudo H, Saga H & Kamada H (1991) Shikonin production and secretion by hairy root cultures ofLithospermum erythrorhizon. Plant Cell Rep. 10: 282–285
Signs MW & Flores HE (1990) The biosynthesis potential of plant roots. BioEssays 12: 7–13
Tanaka N, Hayakawa M, Mano Y, Ohkawa H & Matsui C (1985) Infection of turnip and radish storage roots withAgrobacterium rhizogenes. Plant Cell Rep. 4: 74–77
Taya M, Yoyama A, Kondo O & Kobayashi T (1989 a) Growth characteristics of plant hairy roots and their cultures in bioreactors. J. Chem. Eng. Jpn. 22: 84–89
Taya M, Yoyama A, Nomura R, Kondo O, Matsui C & Kobayashi T (1989 b) Production of peroxidase with horseradish hairy root cells in a two step culture system. J. Ferment. Bioeng. 67: 31–34
Taya M, Mine K, Kino-oka M, Tone S & Ichi T (1992) Production and release of pigments by culture of transformed hairy root of red beet. J. Ferment. Bioeng. 73: 31–36
Tepfer D (1990) Genetic transformation usingAgrobacterium rhizogenes. Physiol. Plant. 79: 140–146
Uozumi N, Kohketsu K, Kondo O, Honda H & Kobayashi T (1991) Fed-batch culture of hairy root using frtuctose as a carbon source. J. Ferment. Beioeng. 72: 457–460
Uozumi N, Kato Y, Nakashimada Y & Kobayashi T (1992 a) Excretion of peroxidase from horseradish hairy root in combination with ion supplementation. Appl. Microbiol. Biotechnol. 37: 560–565
Uozumi N, Nakashimada Y, Kato Y & Kobayashi T (1992 b) Production of artificial seed from horseradish hairy root. J. Ferment. Bioeng. 74: 21–26
Uozomi N, Kohketsu K & Kobayashi T (1993) Plant hiary root growth and metabolism in fed-batch culture on monosaccharide medium. J. Chem. Technol. Biotechnol. 57: 155–161
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uozumi, N., Asano, Y. & Kobayashi, T. Micropropagation of horseradish hairy root by means of adventitious shoot primordia. Plant Cell Tiss Organ Cult 36, 183–190 (1994). https://doi.org/10.1007/BF00037718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037718