Summary
A tomato chloroplast genome map has been constructed with the restriction enzymes Hpa I, Pvu II, and Sal I. Twelve plastid genes have been located on the tomato plastid genome (159 kb).
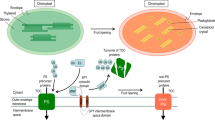
The expression of plastid genes during tomato fruit ripening has been studied. The levels of transcripts of various genes coding for proteins of the photosystem I (psaA), photosystem II (psbA, psbB, psbC, psbD) and the stroma (rbcL) decrease when plastids differentiate from chloroplasts to chromoplasts. The amount of plastid ribosomal RNA also decreases. Transcripts of the genes for the P700 reaction center protein (psaA), for the photosystem II-associated proteins (psbC, psbD) and for the large subunit of ribulose-1,5-bisphosphate carboxylase (rbcL) cannot be detected in chromoplasts. In contrast, a relatively high level of mRNA is present for the 32 kD protein (‘herbicide-binding protein’, psbA) in red fruit.
Similar content being viewed by others
References
Alt J, Morris J, Westhoff P, Herrmann RG: Nucleotide sequence of the clustered genes for the 44 kD chlorophyll a apoprotein and the ‘32 kD’-like protein of the photosystem II reaction center in the spinach plastid chromosome. Current Genetics 8:597–606, 1984.
Apel K, Kloppstech K: The effect of light on the biosynthesis of the light-harvesting chlorophyll a/b protein. Planta 150:426–430, 1980.
Bennet J: Biosynthesis of the light-harvesting chlorophyll a/b protein. Eur J Biochem 118:61–70, 1981.
Ben-Shaul Y, Naftali Y: The development and ultrastructure of lycopene bodies in chromoplasts of Lycopersicon esculentum. Protoplasma 67:333–344, 1969.
Chua N-H, Gilham NW: The sites of synthesis of the principal thylakoid membrane polypeptide in Chlamydomonas reinhardii. J Cell Biol 77:441–452, 1977.
Christofferson RE, Tucker ML, Laties GG: Cellulase gene expression in ripening avocado fruit: The accumulation of cellulase mRNA and protein as demonstrated by cDNA hybridizations and immunodetection. Plant Mol Biol 3:385–391, 1984.
Clewell DB, Helinski DR: Supercoiled circular DNA-protein complex in Escherichia coli: Purification and induced conversion to an open circular DNA form. Proc Natl Acad Sci USA 62:1159–1166, 1969.
Crookes PR, Grierson D: Ultrastructure of tomato fruit ripening and the role of polygalacturonase isoenzymes in the cell wall degradation. Plant Physiol 72:1088–1093, 1983.
Crouse EJ, Schmitt JM, Bohnert H-J, Gordon K, Driesel AJ, Herrmann RG: Intramolecular compositional heterogeneity of Spinacia and Euglena chloroplast DNAs. With an appendix: Bohnert H-J, Gordon K, Driesel AJ, Crouse EJ, Herrmann RG: Mapping of genes on the restriction endonuclease site map of Spinacia oleracea chloroplast DNA. In: Akoyunoglou G, Argyroudi-Akoyunoglou JH (eds) Chloroplast development. Elsevier, Amsterdam, 1978, pp 565–572.
Deno H, Shinozaki K, Sugiura M: Nucleotide sequence of tobacco chloroplast gene for the alpha subunit of proton-translocating ATPase. Nucl Acid Res 11:2185–2191, 1983.
Deno H, Kato A, Shinozaki M: Nucleotide sequences of tobacco chloroplast genes for elongator tRNAMet and tRNAVal(UAC): the tRNAVal(UAC) gene contains a long intron. Nucl Acid Res 10:7511–7520, 1982.
Deno H, Shinozaki K, Sugiura M: Structure and transcription pattern of a tobacco chloroplast gene coding for subunit III of proton-translocating ATPase. Gene 32:195–201, 1984.
Dostal HC, Leopold AC: Gibberellin delays ripening of tomatoes. Science 158:1579–1580, 1967.
Ellis RJ: Chloroplast proteins: synthesis, transport, and assembly. Ann Rev Plant Physiol 32:111–137, 1981.
Grierson D: Electrophoresis of RNA. In: Rickwood D, Hames BD, Grierson D: Electrophoresis of RNA. In: Rickwood D, Hames BD (eds) Gel Electrophoresis of Nucleic Acids: A Practical Approach. IRL press, Oxford, Washington DC, 1982, pp 1–38.
Grierson D, Slater A, Speirs J, Tucker GA: The appearance of polygalacturonase mRNA in tomatoes: one of a series of changes in gene expression during development and ripening. Plant 163:263–271, 1985.
Hall TC: Plant messenger RNA. In: Hall TC, Davies JW (eds) Nucleic Acids in Plants. CRC Press, Boca Roton, Florida, 1979, pp 217–225.
Hallick RB, Richards OC, Gray PW: Isolation of intact, superhelical chloroplast DNA from Euglena gracilis. In: Edelman M, Hallick RB, Chua N-H (eds) Methods in Chloroplast Molecular Biology. Elsevier Biomedical Press, Amsterdam, New York, Oxford, 1982, pp 281–293.
Hausmann P, Sitte P: Comparison of the polypeptide complement of different plastid types and mitochondria of Narcissus pseudonarcissus. Z Naturf 39c:758–766, 1984.
Harpster MH, Mayfield SP, Taylor WC: Effects of pigment-deficient mutants on the accumulation of photosynthetic proteins in maize. Plant Mol Biol 3:59–71, 1984.
Harris WM, Spurr AR: Chromoplasts of tomato fruits: I. Ultrastructure of low-pigment and high-beta mutants: carotene analysis. Amer J Bot 56:369–379, 1969.
Harris WM, Spurr AR: Chromoplasts of tomato fruits: II. The red tomato. Amer J Bot 56:380–389, 1969.
Heinemeyer W, Alt J, Herrmann RG: Nucleotide sequence of the clustered genes for the apocytochrome b6 and subunit 4 of the cytochrome b/f complex in the spinach plastid chromosome. Current Genetics 8:543–549, 1984.
Highfield PE, Ellis RJ: Synthesis and transport of the small subunit of chloroplast ribulose bisphosphate carboxylase. Nature 271:420–424, 1978.
Hoffmann-Falk H, Mattoo AK, Marder JB, Edelmann M, Ellis RJ: General occurrence and structural similarity of the rapidly synthesized, 32 000-dalton protein of the chloroplast membrane. J Biol Chem 257:4583–4587, 1982.
Holschuh K, Bottomley W, Whitfeld PR: Structure of the spinach chloroplast genes for the D2 and 44 kD reaction center proteins of photosystem II and for tRNASer(UGA). Nucl Acid Res 12:8819–8834, 1984.
Humphreys GO, Willshaw GA, Anderson ES: A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta 383:457–463, 1975.
Iwatsuki N, Moriyama R, Asahi T: Isolation and properties of intact chromoplasts from tomato fruits. Plant and Cell Physiol 25:763–768, 1984.
Jorgensen RA, Cuellar RE, Thompson WF: Modes and tempos in the evolution of nuclear-encoded ribosomal RNA genes in legumes. Carnegie Institution of Washington Year Book 81:98–101, 1981–1982.
Kannangasa CG, Gough SP, Hansen B, Rasmussen JN, Simpson DJ: A homogenizer with replaceable razor blades for bulk isolation of active barley plastids. Carlsberg Res Commun 42:431–439, 1977.
Khudairi AK, Arboleda OP: Phytochrome-mediated carotenoid biosynthesis and its influence by plant hormones. Plant Plantarum 24:18–22, 1971.
Khudairi AK, Juglekar R: Phytochrome-mediated abscisic acid synthesis in tomatoes. Plant Physiol 47:11, 1971.
Khudairi AK: The ripening of tomatoes. American Scientists 60:696–707, 1972.
Krebbers ET, Larrinua IM, McIntosh L, Bogorad L: The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucl Acid Res 10:4985–5002, 1982.
Kung SD, Sakano K, Wildman SG: Multiple peptide composition of the large and small subunits of Nicotiana tabacum fraction I protein ascertained by fingerprinting and electrofocusing. Biochim Biophys Acta 365:138–147, 1974.
Link G, Langridge U: Structure of the chloroplast gene for the precursor of the Mt 32 000 photosystem II protein from mustard (Sinapis alba I). Nucl Acid Res 12:945–958, 1984.
Lorimer GH: The carboxylation and oxygenation of ribulose-1,5-bisphosphate: The primary events in photosynthesis and photorespiration. Ann Rev Plant Physiol 32:349–389, 1981.
Maniatis T, Fritsch EF, Sambrook J: Molecular cloning, a laboratory manual. Cold Spring Harbor, 1982.
Mohr WP, Stein M: Fine structure of fruit development in tomato. Can J Plant Sci 49:549–533, 1969.
Morris J, Herrmann RG: Nucleotide sequence of the gene for the P680 chlorophyll a apoprotein of the photosystem II reaction center from spinach. Nucl Acid Res 12:2837–2849, 1984.
Palmer JD, Zamir D: Chloroplast DNA evolution and phylogenetic relationships in Lycopersicon. Proc Natl Acad Sci USA 79:5006–5010, 1982.
Palmer JD, Edwards H, Jorgensen RA, Thompson WF: Novel evolutionary variation in transcription and location of two chloroplast genes. Nucl Acid Res 10:6819–6832, 1982.
Pero J, Hannett NM, Talkington C: Restriction cleavage map of SP01 DNA: General location of early, middle, and late genes. J Virol 31:156–171, 1979.
Rattanapanone N, Grierson D, Stein M: Ribonucleic acid metabolism during the development and ripening of tomato fruits. Phytochemistry 16:629–633, 1977.
Rattanapanone N, Speirs J, Grierson D: Evidence for changes in messenger RNA content related to tomato fruit ripening. Phytochemistry 17:1485–1486, 1978.
Rasmussen OF, Bookjans G, Stummann BH, Henningsen KW: Localization and nucleotide sequence of the gene for the membrane polypeptide D2 from pea chloroplast DNA. Plant Mol Biol 3:191–199, 1984.
Raymundo LC, Chichester CO, Simpson KL: Light-dependent carotenoid synthesis in tomato fruit. J Agric Food Chem 24:59–64, 1976.
Rick CM: Cytogenetics of the tomato. Adv Genet 8:267–382, 1956.
Rick CM: The tomato. In: King RC (ed) Handbook of Genetics, Vol 2. Plenum Press, New York, 1975, pp 247–280.
Rochaix JD, Dron M, Rahire M, Malnoe P: Sequence homology between the 32 K dalton and the D2 chloroplast membrane polypeptide of Chlamydomonas reinhardii. Plant Mol Biol 3:363–370, 1984.
Rosso SW: The ultrastructure of chromoplast development in red tomato. J Ultrastruc Res 25:307–322, 1968.
Shinozaki K, Sugiura M: The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene 20:91–102, 1982.
Shinozaki K, Deno H, Kato A, Sugiura M: Overlap and cotranscription of the genes for the beta and epsilon subunits of tobacco chloroplast ATPase. Gene 24:147–155, 1983.
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517, 1975.
Sugita M, Sugiura M: Nucleotide sequence and transcription of the gene for the 32 000 dalton thylakoid membrane protein from Nicotiana tabacum. Mol Gen Genet 195:308–313, 1984.
Thomson WW, Whatley JM: Development of nongreen plastids. Ann Rev Plant Physiol 31:375–394, 1980.
Westhoff P, Alt J, Nelson N, Bottomley W, Buenemann H, Herrmann RG: Genes and transcripts for the P700 chlorophyll a apoprotein and subunit 2 of the photosystem I reaction center complex from spinach thylakoid membranes. Plant Mol Biol 2:95–107, 1983.
Westhoff P, Alt J, Herrmann RG: Localization of the genes for the two chlorophyll a conjugated polypeptides (mol. wt. 51 and 44 kD) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J 2: 2229–2237, 1983.
Whitfeld PR, Bottomley W: Organization and structure of chloroplast genes. Ann Rev Plant Physiol 34:279–310, 1983.
Zurawski G, Bottomley W, Whitfeld PR: Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci USA 79:6260–6264, 1982.
Zurawski G, Bohnert HJ, Whitfeld PR, Bottomley W: Nucleotide sequence of the gene for the M, 32 000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of Mr 38950. Proc Natl Acad Sci USA 79:7699–7703, 1982.
Zurawski G, Clegg MT: The barley chloroplast DNA atp beta, epsilon, trnM2, and trnV1 loci. Nucl Acid Res 12:2549–2559, 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piechulla, B., Imlay, K.R.C. & Gruissem, W. Plastid gene expression during fruit ripening in tomato. Plant Mol Biol 5, 373–384 (1985). https://doi.org/10.1007/BF00037558
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037558