Abstract
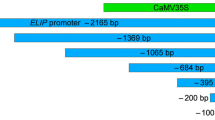
Fruit-specific expression of β-glucuronidase (GUS) activity was produced in transgenic tomato plants when the GUS-coding region was flanked by 5′ and 3′ regions of the tomato 2A11 gene. Deletion studies on the 5′ region revealed a number of strong regulatory elements involved in the proper expression of the 2A11 gene. A 4.0 kb and a 1.3 kb 5′ region can confer high-level fruit-specific GUS expression, while a 1.8 kb 5′ region produces no GUS activity in leaf or fruit tissue. Thus, a strong negative regulatory element is present in the region between 1324 bp and 1796 bp upstream of the 2A11 transcriptional start and a strong fruit-specific positive regulatory element is present more than 1.8 kb upstream of the transcriptional start site. The 1.8 kb promoter region can be activated by the upstream insertion of the CaMV 35S enhancer sequence, albeit not in a fruit-specific fashion. Substitution of the 3′ region of the 2A11 gene with a different 3′ region does not seem to affect GUS expression significantly, indicating a minor role, if any, for the 3′ region in the fruit-specific expression of the 2A11 gene.
Similar content being viewed by others
References
An, G, Mitra, A, Choi, HK, Costa, MA, An, K, Thornburg RW, Ryan, CA: Functional analysis of the 3′ control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell 1: 115–122 (1989).
Barker, RF, Idler, KB, Thompson, DV, Demp, JD: Nucleotide sequence of the T-DNA region from the Agrobacterium tumefaciens octopine Ti plasmid pTi15955. Plant Mol Biol 2: 335–350 (1983).
Benfey, PN, Ren, L, Chua, N-H: Combinational and synergistic properties of CaMV 35S enhancer subdomains. EMBO J 9: 1685–1696 (1990).
Benfey, PN, Ren, L, Chua, N-H: Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J 9: 1677–1684 (1990).
Bradford, MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Broglie, KE, Biddle, P, Cressman, R, Broglie, R: Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic plants. Plant Cell 1: 599–607 (1989).
Callis, J, Fromm, M, Walbot, V: Introns increase gene expression in cultured maize cells. Genes Develop 1: 1183–1200 (1987).
Castresana, C, Garcia-Luque, I, Alonso, E, Malik, VS, Cashmore, AR: Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J 7: 1929–1936 (1988).
Comai, L, Moran, P, Maslyar, D: Novel and useful properties of a chimeric plant promoter combining CaMV 35S and MAS elements. Plant Mol Biol 15: 373–381 (1990).
Cordes, S, Deikman, J, Margossian, LJ, Fischer, RL: Interaction of a developmentally regulated DNA binding factor with sites flanking two different fruit-ripening genes from tomato. Plant Cell 1: 1025–1034 (1989).
Czarnecka, E, Key, JL, Gurley, WB: Regulatory domains of the Gmhsp 17.5-E heat shock promoter of soybean. Mol Cell Biol 9: 3457–3463 (1989).
De, Almeida, ERP, Gossele, V, Muller, CG, Dockx, J, Reynearts A, Botterman, J, Krebbers, E, Timko, MP: Transgenic expression of two marker genes under the control of an Arabidopsis rbcS promoter: sequences encoding the Rubisco transit peptide increase expression levels. Mol Gen Genet 218: 78–86 (1989).
Dean, C, Favreau, M, Bedbrook, J, Dunsmuir, P: Sequences 5′ to translation start regulate expression of petunia rbcS genes. Plant Cell 1: 209–215 (1989).
Dean, C, Favreau, M, Bond-Nutter, D, Bedbrook, J, Dunsmuir P: Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes. Plant Cell 1: 201–208 (1989).
Ecker, JR, Davis, RW: Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84: 5202–5206 (1987).
Fang, R-X, Nagy, F, Sivasubramaniam, S, Chua, N-H: Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell 1: 141–150 (1989).
Fillatti, JJ, Kiser, J, Rose, R, Comai, L: Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Bio/technology 5: 726–730 (1987).
Fluhr, R, Kuhlemeier, C, Nagy, F, Chua, N-H: Organ-specific and light-induced expression of plant genes. Science 232: 1106–1112 (1986).
Gardner, RC, Howarth, AJ, Brown-Luedi, M, Shephard RJ, Messing, J: The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun cloning. Nucl Acids Res 9: 2871–2889 (1981).
Hoekema, A, Hirsch, HR, Hooykaas, PJJ, Schilperoort, RA: A binary vector strategy based on separation of vir and T-region of the Agrobacterium tumefaciens Ti plasmid. Nature 303: 179–181 (1983).
Holsters, M, De, Waele, D, Depicker, A, Messens, E, Van Montagu, M, Schell, J: Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163: 181–187 (1978).
Ingelbrecht, ILW, Herman, LMF, Dekeyser, RA, Van Montagu, MC, Depicker, AG: Different 3′ end regions strongly influence the level of gene expression in plant cells. Plant Cell 1: 671–680 (1989).
Jefferson, RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Keil, M, Sanchez-Serrano, J, Schell, J, Willmitzer, L: Localization of elements important for the wound-inducible expression of a chimeric potato proteinase inhibitor II-CAT gene in transgenic tobacco plants. Plant Cell 2: 61–70 (1990).
Kuhlemeier, C, Cuozzo, M, Green, PJ, Goyvaerts, E, Ward K, Chua, N-H: Localization and conditional redundancy of regulatory elements in rbcS-3A, a pea gene encoding the small subunit of ribulose-bisphosphate carboxylase. Proc Natl Acad Sci USA 85: 4662–4666 (1988).
Kuhlemeier, C, Fluhr, R, Green, P, Chua, N-H: Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Devel 1: 247–255 (1987).
Kuhlemeier, C, Strittmatter, G, Ward, K, Chua, N-H: The pea rbcS-3A promoter mediates light responsiveness but not organ specificity. Plant Cell 1: 471–478 (1989).
Matzke, AJM, Stoger, EM, Schernthaner, JP, Matzke, MA: Deletion analysis of a zein gene promoter in transgenic tobacco plants. Plant Mol Biol 14: 323–332 (1990).
McBride, KE, Summerfelt, KR: Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol Biol 14: 269–276 (1990).
Mitra, A, An, G: Three distinct regulatory elements comprise the upstream promoter region of the nopaline synthase gene. Mol Gen Genet 215: 294–299 (1989).
Odell, JT, Knowlton, S, Lin, W, Mauvais, CJ: Properties of an isolated transcription stimulating sequence derived from the cauliflower mosaic virus 35S promoter. Plant Mol Biol 10: 263–272 (1988).
Ow, WD, Jacobs, JR, Howell, SH: Functional regions of the cauliflower mosaic virus 35S RNA promoter determined by use of the firefly luciferase gene as a reporter of promoter activity. Proc Natl Acad Sci USA 84: 4870–4874 (1987).
Pear, JR, Ridge, N, Rasmussen, R, Rose, RE, Houck, CM: Isolation and characterization of a fruit-specific cDNA and the corresponding clone from tomato. Plant Mol Biol 13: 639–651 (1989).
Radke, SE, Andrews, BM, Moloney, MM, Crouch, ML, Kridl, JC, Knauf, VC: Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75: 685–694 (1988).
Riggs, CD, Voelker, TA, Chrispeels, MJ: Cotyledon nuclear proteins bind to DNA fragments harboring regulatory elements of phytohemagglutinin genes. Plant Cell 1: 609–621 (1989).
Robert, LS, Thompson, RD, Flavell, RB: Tissue-specific expression of a wheat high molecular weight glutenin gene in transgenic tobacco. Plant Cell 1: 569–578 (1989).
Sambrook, J, Fritsch, EF, Maniatis, T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Sanger, F, Nicklen, S, Coulson, AR: DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sheehy, RE, Pearson, J, Brady, CJ, Hiatt, WR: Molecular characterization of tomato fruit polygalacturonase. Mol Gen Genet 208: 30–36 (1987).
Stockhaus, J, Schell, J, Willmitzer, L: Identification of enhancer elements in the upstream region of the nuclear photosynthetic gene ST-LS1. Plant Cell 1: 805–813 (1989).
Thornburg, RW, An, G, Cleveland, TE, Johnson, R, Ryan CA: Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci USA 84: 744–748 (1978).
Tuan, DYH, Solomon, WB, London, IM, Lee, DP: An erythroid-specific developmental-stage-independent enhancer far upstream of the human ‘B-like globin’ genes. Proc Natl Acad Sci USA 86: 2554–2558 (1989).
Ueda, T, Pichersky, E, Malik, VS, Caxhmore, AR: Level of expression of the tomato rbcS-3A gene is modulated by a far upstream promoter element in a developmentally regulated fashion. Plant Cell 1: 217–227 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Haaren, M.J.J., Houck, C.M. Strong negative and positive regulatory elements contribute to the high-level fruit-specific expression of the tomato 2A11 gene. Plant Mol Biol 17, 615–630 (1991). https://doi.org/10.1007/BF00037048
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037048