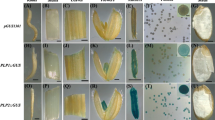
Abstract
We have previously reported the isolation and characterization of a gene (Zm 13) from Zea mays which shows a pollen-specific pattern of expression. Stably transformed tobacco plants containing a reporter gene linked to portions of the Zm 13 5′ flanking region show correct temporal and spatial expression of the gene. Here we present a more detailed analysis of the 5′ regions responsible for expression in pollen by utilizing a transient expression system. Constructs containing the β-glucuronidase (GUS) gene under the control of various sized fragments of the Zm13 5′ flanking region were introduced into Tradescantia and Zea mays pollen via high-velocity microprojectile bombardment, and monitored both visually and with a fluorescence assay. The results suggest that sequences necessary for expression in pollen are present in a region from −100 to −54, while other sequences which amplify that expression reside between −260 and −100. The replacement of the normal terminator with a portion of the Zm13 3′ region containing the putative polyadenylation signal and site also increased GUS expression. While the −260 to −100 region contains sequences similar to other protein-binding domains reported for plants, the −100 to −54 region appears to contain no significant homology to other known promoter fragments which direct pollen-specific expression. The microprojectile bombardment of Tradescantia pollen appears to be a good test system for assaying maize and possibly other monocot promoter constructs for pollen expression.
Similar content being viewed by others
References
Albani D, Robert LS, Donaldson PA, Altosaar I, Arnison PG, Fabijanski SF: Characterization of a pollenspecific gene family from Brassica napus which is activated during early microspore development. Plant Mol Biol 15: 605–622 (1990).
Ferl RJ, Nick H. In vivo detection of regulatory factor binding sites in the 5′ flanking region of maize Adh 1. J Biol Chem 262: 7947–7950 (1986).
Gilmartin P, Sarokin L, Memelink J, Chua N-H: Molecular light switches for plant genes. Plant Cell 2: 369–378 (1990).
Green PJ, Yong M-H, Cuozzo M, Kano-Murakami Y, Silverstein P, Chua N-H. Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene. EMBO J 7: 4035–4044.
Guerrero FD, Crossland L, Smutzer GS, Hamilton DA, Mascarenhas JP. Promoter sequences from a maize pollen-specific gene direct tissue-specific transcription in tobacco. Mol Gen Genet 224: 161–168 (1990).
Guiltinan MJ, Marcotte WR, Quatrano RS: A plant leucine zipper protein that recognizes and abscisic acid response element. Science 250: 267–271 (1990).
Hamilton DA, Bashe DM, Stinson JR, Mascarenhas JP: Characterization of a pollen-specific genomic clone from maize. Sex Plant Reprod 2: 208–212 (1989).
Hanson DD, Hamilton DA, Travis JL, Bashe DM, Mascarenhas JP. Characterization of a pollen-specific cDNA clone from Zea mays and its expression. Plant Cell 1: 173–179 (1989).
Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, Fischhoff DA, Re DB, Fraley R, Horsch RB. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Bio/ technology 6: 915–922 (1988).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Jefferson RA, Kavanagh TA, Bevan MV. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Katagiri F, Lam E, Chua N-H. Two tobacco DNA-binding proteins have homology to CREB. Nature 340: 727–730 (1989).
Kriz AL, Boston RS, Larkins BA: Structural and transcriptional analysis of DNA sequences flanking genes that encode 19 kilodalton zeins. Mol Gen Genet 207: 90–98 (1987).
Kuhlemeier C, Cuozzo M, Green PJ, Goyvaerts E, Ward K, Chua N-H: Localization and conditional redundancy of regulatory elements in rbcS-3A, a pea gene encoding the small subunit of ribulose-bisphosphate carboxylase. Proc Natl Acad Sci USA 85: 4662–4666 (1988).
McKendree WL, Paul A-L, DeLisle AJ, Ferl RJ: In vivo and in vitro characterization of protein interactions with the dyad G-box of the Arabidopsis Adh gene. Plant Cell 2: 207–214 (1990).
Mascarenhas JP: Pollen tube growth and ribonucleic acid synthesis by vegetative and generative nuclei of Tradescantia. Am J Bot 53: 563–569 (1966).
Mascarenhas JP: The male gametophyte of flowering plants. Plant Cell 1: 657–664 (1989).
Mascarenhas JP: Gene activity during pollen development. Annu Rev Plant Physiol Plant Mol Biol 41: 317–338 (1990).
Mascarenhas JP: Male gametophyte expressed genes. J Cell Biochem Suppl. 15A: 32 (1991).
Schernthaner JP, Matzke MA, Matzke AJM: Endosperm-specific activity of a zein gene promoter in transgenic tobacco plants. EMBO J 7: 1249–1255 (1988).
Tanksley SD, Zamir D, Rick CM: Evidence for extensive overlap of sporophytic and gametophytic gene expression in Lycopersicon esculentum. Science 213: 453–455 (1981).
Twell D, Klein TM, Fromm ME, McCormick S: Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol 91: 1270–1274 (1989).
Twell D, Wing RA, Yamaguchi J, McCormick S: Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217: 240–245 (1989).
Twell D, Yamaguchi J, McCormick S: Pollen-specific gene expression in transgenic plants: Coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109: 705–713 (1990).
Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S: Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Devel 5: 496–507 (1991).
van Tunen AJ, Hartman SA, Mur LA, Mol JNM: Regulation of chalcone flavone isomerase (CHI) gene expression in Petunia hybrida: The use of alternative promoters in corolla, anthers and pollen. Plant Mol Biol 12: 539–551 (1989).
van Tunen AJ, Mur LA, Brouns GS, Rienstra J-D, Koes RE, Mol JNM: Pollen- and anther-specific promoters from petunia: Tandem promoter regulation of the chiA gene. Plant Cell 2: 393–401 (1990).
Willing RP, Bashe D, Mascarenhas JP: An analysis of the quantity and diversity of messenger RNAs from pollen and shoots of Zea mays. Theor Appl Genet 75: 751–753 (1988).
Willing RP, Mascarenhas JP: Analysis of the complexity and diversity of mRNAs from pollen and shoots of Tradescantia. Plant Physiol 75: 865–868 (1984).
Ye G-N, Daniell H, Sanford JC: Optimization of delivery of foreign DNA into higher-plant chloroplasts. Plant Mol Biol 15: 809–819 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamilton, D.A., Roy, M., Rueda, J. et al. Dissection of a pollen-specific promoter from maize by transient transformation assays. Plant Mol Biol 18, 211–218 (1992). https://doi.org/10.1007/BF00034950
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034950