Abstract
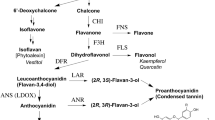
Subclones from a wild carrot cell culture have been examined for their anthocyanin accumulation in the absence and presence of DMSO and 4-coumaric acid, naringenin, dihydroquercetin or leucocyanidin. Subclones that accumulate no or extremely low levels of anthocyanin do not increase their anthocyanin accumulation when treated with DMSO or intermediates. These compounds increased the anthocyanin accumulation in subclones which produce detectable anthocyanin in their absence.
Chalcone synthase was shown to be present in clones and the activity showed no correlation with the amount of anthocyanin accumulated. This suggests that the enzymes of anthocyanin biosynthesis are not coordinately repressed in the subclones which accumulate little or no anthocyanin. Dihydroquercetin and catechin were present in subclones with little or no anthocyanin but no procyanidin was detected which suggests that these subclones biosynthesize leucocyanidin but do not convert it into colorless procyanidins as a major alternative metabolic pathway to anthocyanin biosynthesis. The possibility that some clones are not anthocyanin accumulating because they have impaired transport of the sinapoylated anthocyanin into the vacuole is discussed.
Similar content being viewed by others
References
Belunis CJ & Hrazdina G (1988) Phenylalanine ammonia-lyase from illuminated buckwheat hypocotyls and production of monoclonal antibodies. Phytochem. 27: 2485–2489.
Cheng CL, Wetherell DF & Dougall DK (1985) 4-Coumarate: CoA Ligase in Wild Carrot Cell Culture Clones Which Accumulate Different Amounts of Anthocyanin. In: Neumann et al. (Eds) Primary and Secondary Metabolism of Plant Cell Cultures (pp 87–98). Springer-Verlag Berlin, Heidelberg
Deus-Neumann B & Zenk MH (1984) Instability of indole alkaloid production in Catharanthus roseus cell suspension cultures. Planta Medica 1984: 427–431
Dougall DK (1985) Chemicals from plant cell cultures: yields and variation. In: Zaitlen M, Day P & Hollaender A (Eds) Biotechnology in Plant Science: Relevance to Agriculture in the Eighties (pp 179–190). Academic Press, Orlando, FL
Dougall DK (1987) Cell cloning and the selection of high yielding strains. In: Vasil IK & Constable F (Eds) Cell Culture and Somatic Cell Genetics of Plants, Vol 4, Cell Culture in Phytochemistry (pp 117–123), Academic Press, Orlando, FL
Dougall DK (1989a) Sinapic acid stimulation of anthocyanin accumulation in carrot cell cultures. Plant Science 60: 259–262
Dougall DK (1989b) Dihydroquercetin accumulation in the medium of some wild carrot culture subclones. Plant Cell, Tiss. Org. Cult. 18: 105–112
Dougall DK, Johnson JM & Whitten GH (1980) A clonal analysis of anthocyanin accumulation by cell cultures of wild carrot. Planta 149: 292–297.
Dougall DK, LaBrake S & Whitten GH (1983) Growth and anthocyanin accumulation rates of carrot suspension cultures grown with excess nutrients after semicontinuous culture with different limiting nutrients at several dilution rates, pHs, and temperatures. Biotechnol. Bioeng. 25: 581–594.
Dougall DK & Vogelien DL (1987) The stability of accumulated anthocyanin in suspension cultures of the parental line and high and low accumulating subclones of wild carrot. Plant Cell, Tiss. Org. Cult. 8: 113–123
Dougall DK & Vogelien DL (1990) Anthocyanin yields of clonal wild carrot cell cultures. Effects of serial cloning plus selection for high or low yield (submitted)
Dougall DK & Weyrauch KW (1980) Growth and anthocyanin production by carrot suspension cultures grown under chemostat conditions with phosphate as the limiting nutrient. Biotechnol. Bioeng. 22: 337–352
Ellis BI (1985) Characterization of clonal cultures of Anchusa officinalis derived from single cells of known productivity. J. Plant Physiol. 19: 149–158
Heller W, Britsch L, Forkmann G & Grisebach H (1985) Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Mathiola incana R. Br. Planta 163: 191–196
Hinderer W, Petersen M & Seitz H (1984) Inhibition of flavonoid biosynthesis by gibberellic acid in cell suspension cultures of Daucus carota L. Planta 160: 544–549
Hopp W & Seitz H (1987) The uptake of acylated anthocyanin into isolated vacuoles from a cell suspension culture of Daucus carota. Planta 170: 74–85
Hrazdina G, Lifson E & Weeden NF (1986) Isolation and characterization of buckwheat (Fagopyrum esculentum M.) chalcone synthase and its polyclonal antibodies. Arch. Biochem. Biophys. 247: 414–419
Hrazdina G Wagner GJ & Siegelman HW (1978) Subcellular localization of enzymes of anthocyanin biosynthesis in protoplasts. Phytochem. 17: 53–56
Hrazdina G & Wagner GJ (1985) Metabolic pathways as enzyme complexes: Evidence for synthesis of phenylpropanoids and flavonoids on membrane associated complexes. Arch. Biochem. Biophys. 237: 88–100
Hrazdina GH, Zobel AM & Hoch HC (1987) Biochemical, immunological, and immunocytochemical evidence for the association of chalcone synthase with endoplasmic reticulum membranes. Proc. Natl. Acad. Sci. USA 84: 8966–8970
Kinnersley AM & Dougall DK (1980) Increase in anthocyanin yield from wild-carrot cell cultures by selection system based on cell-aggregate size. Planta 149: 200–204
Kristiansen KN (1984) Biosynthesis of proanthocyanidins in barley: Genetic control of the conversion of dihydroquercetin to catechin and procyanidins. Carlsberg Res. Commun. 49: 503–524
Kristiansen KN (1986) Conversion of (+)-dihydroquercetin to (+)-2,3-trans-3,4-cis-leucocyanidin and (+)-catechin with an enzyme extract from maturing grains of barley. Carlsberg Res. Commun. 51: 51–60
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature (London) 227: 680–685
Meins FJr (1983) Heritable variation in plant cell culture. Ann. Rev. Plant Physiol. 34: 327–346
Porter LJ & Foo LY (1982) Leucocyanidin: synthesis and properties of (2R, 3S, 4R)-(+)-3,4,5,7,3′,4′-hexahydroxyflavan. Phytochem. 21: 2947–2952
Porter L, Hrstich L & Chan B (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochem. 25: 223–230
Tabata M & Fujita Y (1985) Production of shikonin by plant cell cultures. In: Zaitlen M, Day P & Hollaender A (Eds) Biotechnology in Plant Science: Relevance to Agriculture in the Eighties (pp 207–218). Academic Press, Orlando, FL
Towbin H, Staehelin T & Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354
Vogelien DL & Dougall DK (1989) On the nature of the variation in yields of phytochemicals observed within plant cell cultures. In: Kurz WGW (Ed) Primary and Secondary Metabolism of Plant Cell Cultures (pp 53–57). Springer-Verlag, Berlin, Heidelberg
Wetherell DF (1969) Phytochrome in cultured wild carrot tissue. I. Synthesis. Plant Physiol. 44: 1734–1737
Yamada Y & Hashimoto T (1984) Secondary products in tissue culture. In: Collins GB & Petolino JG (Eds) Applications of Genetic Engineering to Crop Improvement (pp 561–604). Martinus Nijhoff/Dr. W. Junk Publishers, Dordrecht
Zenk MH (1978) The impact of plant tissue culture on industry and agriculture. 1. The impact of plant cell culture on industry. In: Thorpe TA (Ed) Frontiers of Plant Tissue Culture (pp 1–14). The International Association for Plant Tissue Culture 1978, The Bookstore, University of Calgary, Calgary, Alberta T2N 1N4, Canada
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vogelien, D.L., Hrazdina, G., Reeves, S. et al. Phenotypic differences in anthocyanin accumulation among clonally related cultured cells of carrot. Plant Cell Tiss Organ Cult 22, 213–222 (1990). https://doi.org/10.1007/BF00033639
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00033639