Abstract
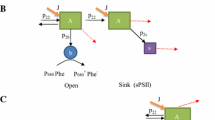
The oxygen flash yield (YO2) and photochemical yield of PS II (ΦPS II) were simultaneously detected in intact Chlorella cells on a bare platinum oxygen rate electrode. The two yields were measured as a function of background irradiance in the steady-state and following a transition from light to darkness. During steady-state illumination at moderate irradiance levels, YO2 and ΦPS II followed each other, suggesting a close coupling between the oxidation of water and QA reduction (Falkowski et al. (1988) Biochim. Biophys. Acta 933: 432–443). Following a light-to-dark transition, however, the relationship between QA reduction and the fraction of PS II reaction centers capable of evolving O2 became temporarily uncoupled. ΦPS II recovered to the preillumination levels within 5–10 s, while the YO2 required up to 60 s to recover under aerobic conditions. The recovery of YO2 was independent of the redox state of QA, but was accompanied by a 30% increase in the functional absorption cross-section of PS II (σPS II). The hysteresis between YO2 and the reduction of QA during the light-to-dark transition was dependent upon the reduction level of the plastoquinone pool and does not appear to be due to a direct radiative charge back-reaction, but rather is a consequence of a transient cyclic electron flow around PS II. The cycle is engaged in vivo only when the plastoquinone pool is reduced. Hence, the plastoquinone pool can act as a clutch that disconnects the oxygen evolution from photochemical charge separation in PS II.
Similar content being viewed by others
Abbreviations
- ADRY:
-
acceleration of the deactivation reactions of the water-splitting enzyme (agents)
- Chl:
-
chlorophyll
- cyt:
-
cytochrome
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- FO :
-
minimum fluorescence yield in the dark-adapted state
- FI :
-
minimum fluorescence yield under ambient irradiance or during transition from the light-adapted state
- FM :
-
maximum fluorescence yield in the dark-adapted state
- FM′ :
-
maximum fluorescence yield under ambient irradiance or during transition from light-adapted state
- FV, FV′ :
-
variable fluorescence (FV=FM–FO ; FV′=FM′–FI)
- FRR:
-
fast repetition rate (fluorometer)
- ΦPS II :
-
quantum yield of QA reduction (ΦPS II=(FM − FO)/FM or ΦPS II)=(FM= − FI=)/FM=)
- LHCII:
-
Chl a/b light harvesting complexes of Photosystem II
- OEC:
-
oxygen evolving complex of PS II
- P680:
-
reaction center chlorophyll of PS II
- PQ:
-
plastoquinone
- POH2 :
-
plastoquinol
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- RC II:
-
reaction centers of Photosystem II
- PS II:
-
the effective absorption cross-section of PHotosystem II
- TL:
-
thermoluminescence
- YO2 :
-
oxygen flash yield
References
Allen J (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335
Allen J, Bennett J, Steinback KE and Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29
Arnon DI (1995) Divergent pathways of photosynthetic electron transfer: The autonomous oxygenic and anoxygenic photosystems. Photosynth Res 46: 47–71
Babcock GT and Sauer K (1973) Electron paramegnetic resonance signal II in spinach chloroplasts I. Kinetic analysis for untreated chloroplasts. Biochim Biophys Acta 325: 483–503
Barber J and De Las Rivas J (1993) A functional model for the role of cytochrome b559 in the protection against donor and acceptor side photoinhibition. Proc Natl Acad Sci USA 90: 10942–10946
Bennoun P (1970) Reoxydation du quencher de fluorescence ‘Q’ en presence de 3-(3,4-dichlorophenyl)-1,1-dimethyluree. Biochim Biophys Acta 216: 357–363
Briantais JM (1994) Light-harvesting chlorophyll a/b complex requirement for regulation of Photosystem II photochemistry by non-photochemical quenching. Photosynth Res 40: 287–294
Briantais JM, Vernotte C, Picaud M and Krause GH (1979) A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta 548: 128–138
Bukhov NG, Mohanty P, Rakhimberdieva MG and Karapetyan NV (1982) Analysis of dark-relaxation kinetics of variable fluorescence in intact leaves. Planta 187: 122–127
Buser CA, Diner BA and Brudvig GW (1992) Photooxidation of cytochrome-b(559) in oxygen-evolving Photosystem-II. Biochemistry 31: 11449–11459
Canaani O and Havaux M (1990) Evidence for a biological role in photosynthesis for cytochrome b-559 a component of Photosystem II reaction center. Proc Natl Acad Sci USA 87: 9295–9299
Crofts AR and Yerkes CT (1994) A molecular mechanism for q(E)-quenching. FEBS Lett 352: 265–270
Crofts AR, Baroli I, Kramer D and Taoka S (1993) Kinetics of electron-transfer between QA and QB in wild-type and herbicideresistant mutants of Chlamydomonas reinhardtii. Z Naturforsch 48C: 259–266
Crofts J and Horton P (1991) Dissipation of excitation energy by Photosystem-II particles at low pH. Biochim Biophys Acta 1058: 187–193
Dau H, Windecker R and Hansen UP (1991) Effect of light-induced changes in thylakoid voltage on chlorophyll fluorescence of Aegopodium podagraria leaves. Biochim Biophys Acta 1057: 337–345
Debus RJ, Barry BA, Babcock GT and McIntosh L (1988) Sitedirected mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci USA 85: 427–430
Delosme R (1967) Etude de l'induction de fluorescence des algues vertes et des chloroplastes au debut d'une illumination intense. Biochim Biophys Acta 143: 108–128
Demmig-Adams B (1990) Carotenoids and photoprotection in plants — a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020: 1–24
Diner B (1975) Dependence of the turnover and deactivation reactions of Photosystem II on the redox state of the pool A varied under anaerobic conditions. In: Avron M (ed) Proceedings of the Third International Congress on Photosynthesis II, pp 589–601. Elsevier, Amsterdam
Diner B (1977) Dependence of the deaetivation reactions of Photosystem II on the redox state of plastoquinone pool A varied under anaerobie conditions. Equilibria on the acceptor side of Photosystem II. Biochim Biophys Acta 460: 247–258
Diner B and Joliot P (1977) Oxygen evolution and manganese. In: Trebst A and Avron M (eds) Encyclopedia of Plant Physiology, New Series, Photosynthesis I, Vol 5, pp 187–205. Springer-Verlag, Berlin
Driesenaar ARJ, Schreiber U and Malkin S (1994) The use of photothermal radiometry in assessing leaf Photosynthesis. 2. Correlation of energy storage to Photosystem II fluorescence parameters. Photosynth Res 40: 45–53
Ducruet JM and Miranda T (1992) Graphical and numerical analysis of thermoluminescence and fluorescence Fo emission in photosynthetic material. Photosynth Res 33: 15–27
Duysens LNM and Sweers HE (1963) Mechanism of the two photochemical reactions in algae as studied by means of fluorescence. In: Japanese Society of Plant Physiologists (eds) Studies on Microalgae and Photosynthetic Bacteria, pp 353–372. University of Tokyo Press, Tokyo
Escoubas JM, Lomas M, LaRoche J and Falkowski PG (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA 92: 10237–10241
Falkowski PG and Kolber Z (1995) Variations in chlorophyll fluorescence yields in phytoplankton in the world oceans. Aust J Plant Physiol 22: 341–355
Falkowski PG, Fujita Y, Ley A and Mauzerall D (1986) Evidence for cyclic electron flow around Photosystem II in Chlorella pyrenoidosa. Plant Physiol 81: 310–312
Falkowski PG, Greene R and Kolber Z (1994) Light utilization and photoinhibition of photosynthesis in marine phytoplankton. In: Baker NR and Bowyer JR (eds) Photoinhibition of Photosynthesis, pp 407–432. Bios Scientific, Oxford
Falkowski PG, Kolber Z and Fujita Y (1988) Effect of redox state on the dynamics of Photosystem II during steady-state photosynthesis in eucaryotic algae. Biochim Biophys Acta 933: 432–443
Falkowski PG, Kolber Z and Mauzerall D (1994) A comment on the call to throw away your fluorescence induction apparatus. Biophys J 66: 923–925
Forbush B and Kok B (1968) Reaction between primary and secondary electron acceptors of Photosystem II of photosynthesis. Biochim Biophys Acta 162: 243–253
Forbush B, Kok B and McGloin M (1971) Cooperation of charges in photosynthetic O2 evolution II. Damping of flash yield oscillation, deactivation. Photochem Photobiol 14: 307–321
France LL, Geacintov NE, Breton J and Valkunas L (1992) The dependence of the degrees of sigmoidicities of fluorescence induction curves in spinach chloroplasts on the duration of actinic pulses in pump-probe experiments. Biochim Biophys Acta 1101: 105–119
Franck F and Schmid GH (1984) Flash pattern of oxygen evolution in greening etioplasts of oat. Z Naturforsch C 39: 1091–1096
Franck F and Schmid GH (1993) Preferential deactivation of the S3 state of the water-oxidizing complex, favoured by plastoquinone reduction in barley chloroplasts. Z Naturforsch 48C: 603–608
Gal A, Hauska G, Herrmann R and Ohad I (1990) Interaction between light harvesting chlorophyll a/b protein (LHC II) kinase and cytochrome b6/f complex. J Biol Chem 265: 19742–19749
Genty B, Briantias JM and Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92
Genty B, Harbinson J, Briantais JM and Baker NR (1990) The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of Photosystem-II photochemistry in leaves. Photosynthe Res 25: 249–257
Ghanotakis DF, Yerkes CT and Babcock GT (1982) The role of reagents accelerating the deactivation reactions of water-splitting enzyme Y (ADRY reagents) in destabilizing high-potential oxidizing equivalents generated in chloroplasts. Biochim Biophys Acta 682: 21–31
Gong H and Ohad I (1991) The PQ/PQH2 ratio and occupancy of Photosystem-II-QB site by plastoquinone. Control the degradation of D1 protein during photoinhibition in vivo. J Biol Chem 266: 2193–21299
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll-a fluorescence. Aust J Plant Physiol 22: 131–160
Harbinson J (1994). The responses of thylakoid electron transport and light utilization efficiency to sink limitation of photosynthesis. In: Baker NR and Bowyer JR (eds) Photoinhibition of Photosynthesis, pp 273–295. Bios Scientific, Oxford
Heber U, Kirk MR and Boardman NK (1979) Photoreactions of cytochrome b-559 and cyclic electron flow in Photosystem II of intact chloroplasts. Biochim Biophys Acta 546: 292–306
Horton P and Black MT (1981) Light dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5-triphosphate. Biochim Biophys Acta 635: 53–62
Horton P, Ruban AV and Walters RG (1994) Regulation of light harvesting in green plants—Indication by nonphotochemical quenching of chlorophyll fluorescence. Plant Physiol 106: 415–420
Joliot P (1965) Etudes simultanées des cinetiques de fluorescence et d'émission d'oxygéne photosynthetique. Biochim Biophys Acta 102: 135–148
Joliot P, Joliot A, Bouges B and Barbieri G (1971) Studies of system II photocenters by comparative measurements of luminescence, fluorescence and oxygen emission. Photochem Photobiol 14: 287–305
Koike H, Siderer Y, Ono T and Inoue Y (1986) Assignment of thermoluminescence A band to S3QA - charge recombination: Sequential stabilization of S3 and QA - by a two-step illumination at different temperatures. Biochim Biophys Acta 850: 80–89
Kok B, Forbush B and McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution- 1. A linear four step mechanism. Photochem Photobiol 11: 457–475
Kolber Z, Zehr J and Falkowski P (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in Photosystem II. Plant Physiol 88: 923–929
Krause GH and Weis E (1991) Chlorophyll fluorescence and photosynthesis—the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349
Lao K, Franzen S, Stanley R, Lambright D and Boxer S (1993) Effects of applied electric-fields on the quantum yields of the initial electron-transfer steps in bacterial photosynthesis. 1. Quantum yield failure. J Phys Chem 97: 13165–13171
Lapointe L, Huner NPA, Leblanc RM and Carpentier R (1993) Possible photoacoustic detection of cyclic electron transport around Photosystem II in photoinhibited thylakoid preparations. Biochim Biophys Acta 1142: 43–48
Laverel J (1975) Luminescence. In: Govindjec (ed) Biocnergetics of Photosynthesis, pp 223–317. Academic Press, New York
Lemasson C and Barbieri G (1971) Effect de la longueur d'onde de prellumination sur la desactivation des formes oxydées du donneur d'electrons du Photosystéme II. Biochim Biophys Acta 245: 386–397
Ley AC and Mauzerall DC (1982) Absolute absorption cross-sections for Photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. Biochim Biophys Acta 680: 95–106
Malkin S and Kok B (1966) Fluorescence induction studies in isolated chloroplasts. Biochim Biophys Acta 126: 413–432
Mauzerall DC (1978) Multiple excitation and the yield of chlorophyll a fluorescence in photosynthetic system. Photochem Photobiol 28: 991–998
Messinger J and Renger G (1994) Analyses of pH-induced modifications of the period four oscillation of flash-induced oxygen evolution reveal distinct structural changes of the Photosystem II donor side at characteristic pH values. Biochemistry 33: 10896–10905
Nugent JHA, Dematriou C and Lockett CJ (1987) Electron donation in Photosystem II. Biochim Biophys Acta 894: 534–542
Olaizola M, Laroche J, Kolber Z and Falkowski PG (1994) Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth Res 41: 357–370
Radmer R and Kok B (1973) A kinetic analysis of the oxidizing and reducing sides of the O2-evolving system of photosynthesis. Biochim Biophys Acta 314: 28–41
Renger G, Bouges-Bocquet B and Delosme R (1973) Studies on the ADRY agent-induced mechanism of the discharge of the holes trapped in the photosynthetic watersplitting enzyme system Y. Biochim Biophys Acta 292: 796–807
Robinson HH and Crofts AR (1983) Kinetics of the oxidation-reduction reactions of the Photosystem II quinone acceptor complex, and the pathway for deactivation. FEBS Lett 153: 221–226
Rutherford AW, Crofts AR and Inoue Y (1982) Thermoluminescence as a probe of Photosystem II photochemistry. The origin of the flash-induced glow peaks. Biochim Biophys Acta 682: 457–465
Rutherford AW and Inoue Y (1984) Oscillations of delayed luminescence from Photosystem II: Recombination of S2QB - and S3QB - FEBS Lett 165: 163–170
Scherer S (1990) Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biochem Sci 15: 458–462
Schreiber U, Schliwa U and Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62
Stiehl HH and Witt HT (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z Natuforsch 24B: 1588–1598
Sundblad LG, Schroder WP and Akerlund HE (1988) S-state distribution and redox state of QA in barley in relation to luminescence decay kinetics. Biochim Biophys Acta 973: 47–52
Thompson LK and Brudvig GW (1988) Cytochrome b-559 may function to protect Photosystem II from photoinhibition. Biochemistry 27: 6653–6658
Thompson LK, Miller AF, de Paula JC and Brudvig GW (1988) Electron donation in Photosystem II. Isr J Chem 28: 121–128
Van Best JA and Duysens LNM (1975) Reaction between primary and secondary acceptors of Photosystem II in Chlorella pyrenoidosa under anaerobic conditions as studied by chlorophyll a fluorescence. Biochim Biophys Acta 408: 154–163
Vass I and Inoue Y (1992) Thermoluminescence in the study of Photosystem II. In: Barber J (ed) Topics in Photosynthesis, The Photosystems: Structure, Function and Molecular Biology, Vol 11, pp 259–294, Elsevier, Amsterdam
Vass I and Styring S (1991) pH-dependent charge equilibria between tyrosine-D and the S-states in Photosystem II. Estimation of relative midpoint redox potentials. Biochemistry 30: 830–839
Vass I, Deak Z, Jegerschold C and Styring S (1990) The accessory electron donor tyrosine-D of Photosystem-II is slowly reduced in the dark during low-temperature storage of isolated thylakoids. Biochim Biophys Acta 1018: 41–46
Vermaas WFJ, Rutherford AW and Hansson O (1988) Site-directed mutagenesis in Photosystem II of the cyanobacterium Synechocystic sp. PCC 6803: Donor D is a tyrosine residue in the D2 protein. Proc Natl Acad Sci USA 85: 8477–8481
Vernotte C, Etienne AL and Briantais JM (1979) Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta 545: 519–527
Whitmarsh J and Cramer WA (1977) Kinetics of the photoreduction of cytochrome b-559 by Photosystem II in chloroplasts. Biochim Biophys Acta 460: 280–289
Whitmarsh J and Cramer WA (1978) A pathway for the reduction of cytochrome b-559 by Photosystem II. Biochim Biophys Acta 501: 83–93
Whitmarsh J and Pakrasi H (1996) Form and Function of cytb559. In: ort D and Yocum CF (eds) Advances in Photosynthesis, Vol 4, Oxygenic Photosynthesis: Light Reactions (in press)
Author information
Authors and Affiliations
Additional information
The US Government right to retain a non-exclusive, royalty free licence in and to any copyright is acknowledged.
Rights and permissions
About this article
Cite this article
Prasil, O., Kolber, Z., Berry, J.A. et al. Cyclic electron flow around Photosystem II in vivo . Photosynth Res 48, 395–410 (1996). https://doi.org/10.1007/BF00029472
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00029472