Abstract
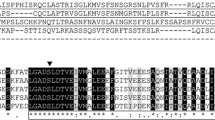
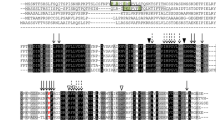
The level of two thioesterases, acyl-CoA thioesterase and acyl-ACP thioesterase was determined during seed maturation in oil seed rape. Both thioesterase activities rose markedly prior to the onset of lipid accumulation, but the induction kinetics suggest that the activities reside on distinct polypeptides. Acyl-ACP thioesterase (EC 3.1.2.14) was purified 2000-fold using a combination of ion exchange, ACP-affinity chromatogr aphy, chromatofocusing and gel filtration. Using native gel electrophoresis, and assays for enzymic activity, two polypeptides were identified on SDS-PAGE as associated with the activity. Cleveland mapping of these polypeptides, of 38 kDa component and 33 kDa respectively, demonstrated that they are related. An antibody was prepared against the 38 kDa component, and this also recognises the 33 kDa polypeptide in highly purified preparations. Western blotting of a crude extract identifies one band at 38 kDa consistent with the 33 kDa component being a degradation product generated during purification. The native molecule has a Mr of 70 kDa indicating a dimeric structure. The enzyme has a pH optimum of 9.5 and shows strong preference for oleoyl-ACP as substrate. The intact enzyme has an N-terminus blocked to protein sequencing. We also found that two other polypeptides co-purify with acyl-ACP thioesterase under native conditions. The N-terminal amino-acid sequence of these polypeptides is shown and their possible identity is discussed.
Similar content being viewed by others
References
Barnes EM: Long chain fatty acyl-thioesterases I and II from Escherichia coli. Meth Enzymol 35: 102–109 (1975).
Bolton P, Harwood JL: Fatty acid biosynthesis by a particulate preparation from germinating pea. Biochem J 168: 261–269 (1977).
Burnette WN: ‘Western blotting’: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112: 195–203 (1981).
Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK: Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem 252: 1102–1106 (1977).
Cottingham IR, Austin A, Sidebottom C, Slabas AR: Purified enoyl-[acyl carrier protein] reductase from rape seed (Brassica napus) contains two closely related polypeptides which differ by a six-amino-acid N-terminal extension. Biochim Biophys Acta 954: 201–207 (1988).
Davies HM, Anderson L, Fan C, Hawkins DJ: Developmental induction, purification, and further characterization of 12:0-ACP thioesterase from immature cotyledons of Umbellularia californica. Arch Biochem Biophys 290: 37–45 (1991).
Guerra DJ, Ohlrogge JB, Frentzen M: Activity of acyl carrier protein isoforms in reactions of plant fatty acid metabolism. Plant Physiol 82: 448–453 (1986).
Harwood JL, Stumpf PK: Fat metabolism in higher plants. LI. Palmitic and stearic synthesis by an avocado supernatant system. Arch Biochem Biophys 148: 282–290 (1972).
Kater MM, Koningstein GM, Nijkamp JJ, Stuitje AR: cDNA cloning and expression of Brassica napus enoylacyl carrier protein reductase in Escherichia coli. Plant Mol Biol 17: 895–909 (1991).
Knudsen J, Clark S, Dils R: Purification and some properties of a medium-chain acyl-thioester hydrolase from lactating-rabbit mammary gland which terminates chain elongation in fatty acid synthesis. Biochem J 160: 683–691 (1976).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Libertini LJ, Smith S: Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem 253: 1393–1401 (1978).
Lin CY, Smith S: Properties of the thioesterase component obtained by limited trypsinization of the fatty acid synthetase multienzyme complex. J Biol Chem 253: 1954–1962 (1978).
Löhden I, Frentzen M: Role of plastidial acyl-acyl carrier protein: glycerol 3-phosphate acyl transferase and acyl-acyl carrier protein hydrolase in channelling the acyl flux through the prokaryotic and eukaryotic pathway. Planta 176: 506–512 (1988).
MacKintosh RW, Hardie DG, Slabas AR: A new assay procedure to study the induction of β-ketoacyl-ACP synthase I and II, and the complete purification of β-ketoacyl-ACP synthase I from developing seeds of oil seed rape (Brassica napus). Biochim Biophys Acta 1002: 114–124 (1989).
Majerus PW, Alberts AW, Vagelos PR: Acyl carrier protein from Escherichia coli. Meth Enzymol 14: 43–50 (1969).
Matsudaira P: Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262: 10035–10038 (1987).
McKeon TA, Stumpf PK: Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem 257: 12141–12147 (1982).
Morrissey JH: Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem 117: 307–310 (1981).
Nakamura Y, Yamada M: Long chain fatty acid synthesis in developing castor bean seeds. I. The operation of the path from acetate to long chain fatty acids in a subcellular particulate system. Plant Cell Physiol 15: 37–48 (1974).
Norton G, Harris JF, Tomlinson A: In: Norton G (ed) Plant proteins, pp. 59–80. Butterworth London (1976).
Ohlrogge JB, Kuhn DN, Stumpf PK: Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci USA 76: 1194–1198 (1979).
Ohlrogge JB, Shine WE, Stumpf PK: Fat metabolism in higher plants — Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys 189: 382–391 (1978).
Olmsted JB: Affinity purification of antibodies from diazotized paper blots of heterogenous protein samples. J Biol Chem 256: 11955–11957 (1981).
Pedersen TA: Lipid formation in Cryptococcus terricolus. III. Extraction and purification of lipids. Acta Chim Scand 16: 374–382 (1962).
Rock CO, Cronan JE: Solubilization, purification and salt activation of acyl-acyl carrier protein synthetase from Escherichia coli. J Biol Chem 254: 7116–7122 (1979).
Rock CO, Garwin JL: Preparative enzymatic synthesis and hydrophobic chromatography of acyl-acyl carrier protein. J Biol Chem 254: 7123–7128 (1979).
Rogers L, Kolattukudy PE, deRenobales M: Purification and characterization of S-acyl fatty acid synthase thioester hydrolase which modifies the product specificity of fatty acid synthase in the uropygial gland of mallard. Biol Chem 257: 880–886 (1982).
Roughan G, Nishida I: Concentrations of long-chain acyl-acyl carrier proteins during fatty acid synthesis by chloroplasts isolated from pea (Pisum salvatum), safflower (Corthamus tinctoris) and amaranthus (Amaranthus lividus) leaves. Arch Biochem Biophys 276: 38–46 (1990).
Seay T, Lueking DR: Purification and properties of acyl coenzyme A thioesterase II from Rhodopseudomonas sphaeriodes. Biochemistry 25: 2480–2485 (1986).
Sheldon PS: Characterization of 3-oxo-acyl[ACP] reductase from Avocado and Brassica napus. Ph.D. thesis (1988).
Sheldon PS, Kekwick RGO, Sidebottom C, Smith CG, Slabas AR: 3-Oxoacyl-(acyl-carrier protein) reductase from avocado (Persea americana) fruit mesocarp. Biochem J 271: 713–720 (1990).
Sheldon PS, Safford R, Slabas AB, Kekwick RGO: 3-oxoacyl-[acyl carrier protein] reductase: a component of plant fatty acid synthase. Biochem Soc Trans 16: 392–393 (1988).
Slabas AR, Aitken A, Howell S, Welham K, Sidebottom CM: Identification of the N-terminal blocking group of rat mammary gland acyl fatty acid synthetase-thioesterase II. Biochem Soc Trans 17: 886–887 (1989).
Slabas AR, Cottingham IR, Austin A, Hellyer A, Safford R, Smith C: Immunological detection of NADH-specific enoyl-[ACP] reductase from rape seed (Brassica napus)—Induction, relationship of α and β polypeptides, mRNA translation and interaction with ACP. Biochim Biophys Acta 1039: 181–188 (1990).
Slabas AR, Harding J, Hellyer A, Roberts P, Bambridge HE: Induction, purification and characterization of acyl carrier protein from developing seeds of oil seed rape (Brassica napus). Biochim Biophys Acta 921: 50–59 (1987).
Slabas AR, Hellyer A: Rapid purification of a high molecular weight subunit polypeptide form of rape seed acetyl CoA carboxylase. Plant Sci 39: 177–182 (1985).
Slabas AR, Sidebottom CM, Hellyer A, Kessell RMJ, Tombs MP: Induction, purification and characterization of NADH-specific enoyl acyl carrier protein reductase from developing seeds of oil seed rape (Brassica napus). Biochim Biophys Acta 877: 271–280 (1986).
Slabas AR, Smith CG: Immunogold localization of acyl carrier protein in plants and Escherichia coli. Planta 175: 145–152 (1988).
Szewczyk B, Summers DF: Preparative elution of proteins blotted to immobilon membranes. Anal Biochem 168: 48–53 (1988).
Tarr GE: In: Shiveley JE (ed) Methods of protein microcharacterization, pp. 155–194. Humana Press, Clifton, NY (1986).
Turnham E, Northcote DH: Changes in the activity of acetyl-CoA carboxylase during rape seed formation. Biochem J 212: 223–229 (1983).
Vick B, Beevers H: Fatty acid synthesis in the endosperm of young castor been seedlings. Plant Physiol 62: 173–178 (1978).
Wessel D, Flügge UI: A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138: 141–143 (1984).
Zilkey BF, Canvin DT: Localization of oleic acid biosynthesis enzymes in the proplastids of developing castor endosperm. Can J Bot 50: 323–326 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hellyer, A., Leadlay, P.F. & Slabas, A.R. Induction, purification and characterisation of acyl-ACP thioesterase from developing seeds of oil seed rape (Brassica napus). Plant Mol Biol 20, 763–780 (1992). https://doi.org/10.1007/BF00027148
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00027148