Abstract
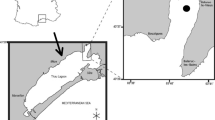
Bacterial and phytoplankton cell number and productivity were measured in the mixolimnion and chemocline of saline meromictic Mahoney Lake during the spring (Apr.–May) and fall (Oct.) between 1982 and 1987. High levels of bacterial productivity (methyl 3H-thymidine incorporation), cell numbers, and heterotrophic assimilation of 14C-glucose and 14C-acetate in the mixolimnion shifted from near surface (1.5 m), at a secondary chemocline, to deeper water (4–7 m) as this zone of microstratification gradually weakened during a several year drying trend in the watershed. In the mixolimnion, bacterial carbon (13–261 µgC 1−1) was often similar to phytoplankton carbon (44–300 µgC 1−1) and represented between 14–57% of the total microbial (phytoplankton + bacteria) carbon depending on the depth interval. Phototrophic purple sulphur bacteria were stratified at the permanent primary chemocline (7.5–8.3 m) in a dense layer (POC 250 mg 1−1, bacteriochlorophyll a 1500–70001µ 1−1), where H2S changed from 0.1 to 2.5 mM over a 0.2 m depth interval. This phototrophic bacterial layer contributed between 17–66% of the total primary production (115–476 mgC m−2 d−1) in the vertical water column. Microorganisms in the phototrophic bacterial layer showed a higher uptake rate for acetate (0.5–3.7 µC 1−1 h−1) than for glucose (0.3–1.4 µgC 1−1 h−1) and this heterotrophic activity as well as bacterial productivity were 1 to 2 orders of magnitude higher in the dense plate than in the mixolimnetic waters above. Primary phytoplanktonic production in the mixolimnion was limited by phosphorus while light penetration appeared to regulate phototrophic productivity of the purple sulphur bacteria.
Similar content being viewed by others
References
American Public Health Association, American Water Works Association & Water Pollution Control Federation, 1985. Standard methods for the examination of water and wastewater, 16th ed. APHA, Wash. D.C. 1268 pp.
Anderson, G. C., 1958. Some limnological features of a shallow saline meromictic lake. Limnol. Oceanogr. 3: 259–270.
Braunegg, G., B. Sonnleitner & R. M. Lafferty, 1978. A rapid gas chromatographic method for the determination of poly-hydroxybutyric acid in microbial biomass. Biotechnol. 6: 29–37.
Cloern, J. E., B. E. Cole & R. S. Oremland, 1983. Autotrophic processes in meromictic Big Soda Lake, Nevada. Limnol. Oceanogr. 28: 1049–1061.
Cloern, J. E., B. E. Cole & S. M. Wienke, 1987. Big Soda Lake (Nevada). 4. Vertical fluxes of particulate matter: Seasonality and variations across the chemocline. Limnol. Oceanogr. 32: 815–824.
Cohen, Y., W. E. Krumbein & M. Shilo, 1977. Solar lake (Sinai) 2. Distribution of photosynthetic microorganisms and primary production. Limnol. Oceanogr. 22: 609–620.
Comeau, Y., W. K. Oldham & K. J. Hall, 1987. Dynamics of carbon reserves in biological dephosphatation of wastewater. Adv. in Water Pollut. Control. 39#x2013;55 IAWPRC Int. Conf. in Rome, Sept. 1987.
Croome, R. L. & P. A. Tyler, 1984. Microbial microstratification and crepuscular photosynthesis in meromictic Tasmanian lakes. Verh. int. Ver. Limnol. 22: 1216–1223.
Culver, D. A. & G. J. Brunskill, 1969. Fayetteville Green Lake v. Studies of primary production and zooplankton in a meromictic marl lake. Limnol. Oceanogr. 14: 862–873.
Dawes, E. A. & P. J. Senior, 1973. The role and regulation of energy reserve polymers in microorganisms. Adv. Microbial Physiol. 10: 135–266.
Fuhram, J. A. & F. Azam, 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: Evaluation of field results. Mar. Biol. 66: 109–120.
Fuhram, J. A., H. W. Ducklow, D. L. Kirchman, J. Hudak, G. B. McManus & J. Kramer, 1986. Does adenine incorporation into nucleic acids measure total microbial production? Limnol. Oceanogr. 31: 627–636.
Griffiths, R. P., S. S. Hayasaka, T. M. McNamara & R. Y. Morita, 1977. Comparison between two methods of assaying relative microbial activity in marine environments. Appl. envir. Microbiol. 34: 801–805.
Guerrero, R., E. Montesinos, C. Pedros-Alio, I. Esteves, J. Mas, H. van Gemerden, P. A. G. Hofman & J. F. Bakker, 1985. Phototrophic sulfur bacteria in two Spanish lakes: Vertical distribution and limiting factors. Limnol. Oceanogr. 30: 919–931.
Hammer, U. T., R. C. Haynes, J. M. Heseltine & S. M. Swanson, 1975. The saline lakes of Saskatchewan. Verh. int. Ver. Limnol. 19: 589–598.
Hayden, J. F., 1972. A limnological investigation of a meromictic lake (Medicine Lake, South Dakota), M. Sc., Univ. of South Dakota, Vermillion.
Hobbie, J. E., R. J. Daley & S. Jasper, 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. envir. Microbiol. 33: 1225–1228.
Hudec, P. P. & P. Sonnenfeld,1980. Comparison of Caribbean solar ponds with inland solar lakes of British Columbia. in A. Nissenbaum (ed.) Hypersaline brines and evaporitic environments. Elsevier, Amsterdam, 101–114.
Lawrence, J. R., R. C. Haynes & U. T. Hammer, 1978. Contribution of photosynthetic green sulphur bacteria to total primary production in a meromictic saline lake. Verh. int. Ver. Limnol. 20: 201–207.
Lovell, C. R. & A. Konopka, 1985. Seasonal bacterial production in a dimictic lake as measured by increases in cell numbers and thymidine incorporation. Appl. envir. Microbiol. 49: 492–500.
Murphy, T. P., K. J. Hall & I. Yesaki, 1983. Coprecipitation of phosphorus with calcite in a naturally eutrophic lake. Limnol. Oceanogr. 28: 58–69.
Northcote, T. G. & T. G. Halsey, 1969. Seasonal changes in the limnology of some meromictic lakes in southern British Columbia. J. Fish Res. Bd, Can. 26: 1763–1787.
Northcote, T. G. & K. J. Hall, 1983. Limnological contrasts and anomalies in two adjacent saline lakes. Hydrobiologia 105: 179–194.
Northcote, T. G. & K. J. Hall,MS. Vernal microstratification patterns in a meromictic saline lake: their causes and biological significance. Hydrobiologia
Parkin, T. B. & T. D. Brock, 1980. Photosynthetic bacterial production in lakes: The effect of light intensity. Limnol. Oceanogr. 25: 711–718.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identification and counting aquatic microflora. Limnol. Oceanogr. 25: 943–948.
Priscu, J. C., R. P. Axler, R. G. Carlton, J. E. Reuter, P. A. Arneson & C. R. Goldman, 1982. Vertical profiles of primary productivity biomass and physiochemical properties in meromictic Big Soda Lake, Nevada, USA. Hydrobiologia 96: 113–120.
Riemann, B., J. A. Fuhram & F. Azam, 1982. Bacterial secondary production in freshwater measured by 3H-thymidine incorporation method. Microb. Ecol. 8: 101–114.
Scavia, D. & G. A. Laird, 1987. Bacterioplankton in Lake Michigan: Dynamics, controls, and significance to carbon flux. Limnol. Oceanogr. 32: 1017–1033.
Strickland, J. H. & T. R. Parsons, 1972. A practical handbook of seawater analysis, 2nd ed. Bull. Fish. Res. Bd, Can. 167.
Takahashi, M. & S. Ichimura, 1968. Vertical distribution and organic matter production of photosynthetic sulfur bacteria in Japanese Lakes. Limnol. Oceanogr. 13: 644–655.
Takahashi, M. & S. Ichimura, 1970. Photosynthetic properties and growth of photosynthetic sulphur bacteria in lakes. Limnol. Oceanogr. 15: 929–944.
van Gemerden, H. & H. H. Beeftink, 1983. Ecology of phototrophic bacteria. in J. G. Ormerod (ed.). The phototrophic bacteria, Studies in Microbiol., 4: 146–179, Univ. of Cal. Press, Berkley.
van Gemerden, H., E. Montesinos, J. Mas & R. Guerrero, 1985. Diel cycle of metabolism of phototrophic purple sulfur bacteria in Lake Cisó (Spain). Limnol. Oceanogr. 30: 932–943.
Veldhius, M. J. W. & H. van Gemerden, 1986. Competition between purple and brown bacteria in a stratified lake: Sulfide, acetate, and light as limiting factors. FEMS Microbial Ecol. 38: 31–38.
Wetzel, R. G., 1973. Productivity investigations of interconnected lakes I. The eight lakes of the Oliver and Walters chains, northeastern Indiana. Hydrobiol. Stud. 3: 91–143.
Wetzel, R. G.,1975.Limnology, W. B. Saunders Co. Toronto. 743 pp.
Wetzel, R. G. & G. E. Likens, 1979. Limnological analyses. W. B. Saunders Co. Philadelphia. 357 pp.
Wood, L. W., 1985. Chloroform-methanol extraction of chlorophyll a. Can. J. Fish. aquat. Sci. 42: 38–43.
Zehr, J. P., R. W. Harvey, R. S. Oremland, J. E. Cloern, L. H. George & J. L. Lane, 1987. Big Soda Lake (Nevada). I. Pelagic bacterial heterotrophy and biomass. Limnol. Oceanogr. 32: 781–793.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hall, K.J., Northcote, T.G. Production and decomposition processes in a saline meromictic lake. Hydrobiologia 197, 115–128 (1990). https://doi.org/10.1007/BF00026944
Issue Date:
DOI: https://doi.org/10.1007/BF00026944